Opiates answers

 Answers to questions on Opiates
Answers to questions on Opiates
Answers to Opiates questions
1. (a) Diesterification (Accept esterification or condensation).
(b) Ethanoic anhydride (it works better than ethanoic acid as it is a stronger nucleophile).
(c) Concentrated sulfuric acid.
(d) More needs to be taken in order to achieve the original effects. The danger is that the lethal dose may be exceeded.
(e) To prevent diseases (e.g. HIV/AIDS or hepatitis) being communicated between one user and another (this can occur if syringes are shared and blood is transferred in the process).
2. Heroin contains two non-polar ester groups which have replaced the more polar hydroxyl, –OH, groups in morphine. Because it is less polar it is more soluble in lipids and is able to penetrate the lipid-based blood/brain barrier and have a quicker and stronger effect on the central nervous system so it is a stronger, and quicker acting, painkiller.
3. (a) To increase its bioavailability. Although morphine contains two polar hydroxyl (–OH) groups it is largely a non-polar molecule due to the ether group and the large amount of hydrocarbon content. To increase its solubility in an aqueous medium the basic amine group can be protonated by hydrochloric acid to make a substituted ammonium ion.
(b)
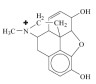
(although the positive charge is shown on the nitrogen atom, in reality it will most likely exist as a complex ion where the positive charge is delocalised over the whole of the molecular ion.)
4. The hydroxyl group attached to the phenyl ring is slightly acidic and will dissociate to form a proton and leave the oxygen atom with a negative charge. Iodomethane is a polar molecule with the δ+ charge on the carbon atom (as iodine is more electronegative than carbon and the C−I bond is much weaker and easier to break than a C−F bond, for example). This is attracted to the negatively charge on the oxygen atom of the hydroxyl group attached to the phenyl group after it has lost a proton. The reaction is nucleophilic substitution.
Download the Answers to Opiates questions ![]()

 IB Docs (2) Team
IB Docs (2) Team