MC test: Nuclear fusion & nuclear fission reactions

 Multiple choice test on C.3 Nuclear fusion & nuclear fission reactions
Multiple choice test on C.3 Nuclear fusion & nuclear fission reactions
Use the following 'quiz' to test your knowledge and understanding of this sub-topic. As this relates to a sub-topic on the options you may need access to the IB data booklet.
If you get an answer wrong, read through the explanation carefully to learn from your mistakes.
When a radioactive isotope undergoes β-decay what particle is always emitted?
During β-decay an electron, 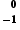 e, is always emitted from the nucleus.
e, is always emitted from the nucleus.
What happens to the mass and atomic number of an element when it loses an α- particle?
An alpha particle is a helium nucleus,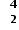 He, so the mass decreases by four and the atomic number decreases by two.
He, so the mass decreases by four and the atomic number decreases by two.
What is X when U-235 nuclei undergo nuclear fission when bombarded with neutrons?
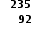 U +
U + 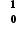 n →
n → 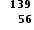 Ba +
Ba +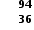 Kr + 3 X
Kr + 3 X
Three neutrons are formed which means a chain reaction can occur as these can go on to react with more U-235 nuclei.
When plutonium-239 reacts with one neutron it produces three neutrons, strontium-90 and an isotope of one other element. What is the identity of this element?
Neutrons have no effect on the total number of protons in a fission reaction. Since 38 of the 94 protons in plutonium (Ar = 94) have gone to make strontium (Ar = 38) the remaining 56 protons must form barium (Ar= 56).
It was recently announced that gold and silver were observed being formed during a kilonova explosion caused by two neutron stars colliding 130 million years ago in a distant galaxy. What technique was used to make this observation?
The light arriving from the explosion was analysed by absorption spectroscopy as each element present could be identified from its unique absorption spectrum.
A sample of protactinium-234 is depleted to 1/32 of its original mass after 5.85 minutes. What is the half-life of Pa-234?
A depletion to 1/32 of the original mass occurs after exactly 5 half-lives so the time for one half-life is (5.85 ÷ 5) minutes.
10.0 g of a 160 g sample of thorium-231 were found to be remaining after 98.4 hours. What is the half-life of Th-231?
10 g is 1/16th of 160 g so 4 half-lives have occurred in 98.4 hours so each half-life is 24.6 hours.
What does the energy (in joules) associated with the frequency of the point at which the lines converge in the ultraviolet region in the absorption spectrum of helium correspond to?
It corresponds to the energy required to promote an electron from the n = 1 to the n = infinity energy level which is the same as the ionization energy of helium for a single electron.
The half-life of strontium-90 is 27.0 years. What percentage of the original mass of a sample of strontium-90 will be present after 108 years?
Four half-lives have passed after 108 years so 1/16th of the mass of the original sample will be remaining which is equal to 6.25%.
The half-life for an radioactive isotope can be determined using the expression t1/2 = ln2/λ. What does λ represent in this equation?
Lambda (λ) is the decay constant which can be obtained from the expression N = Noe−λt where No is the original number of atoms of the isotope present and N is the number of atoms of the isotope present after time t.

 IB Docs (2) Team
IB Docs (2) Team