Proteins & enzymes answers

 Answers to questions on Proteins & enzymes
Answers to questions on Proteins & enzymes
Answers to Proteins & enzymes questions.
1. (a)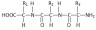
where R1 = –CH2 –COOH , R2 = – CH2–CH2–COOH and R3= –CH(CH3)–OH in any order
(b) Five (six in total).
2. The secondary structure describes the way in which chains of polypeptides fold themselves due to intramolecular hydrogen bonding (this can be either alpha-helix or beta-pleated). The tertiary structure describes the overall folding of the chains by interactions between distant amino acids to give the protein its three-dimensional shape.
3. (i) London dispersion forces
(ii) ionic bonding
(iii) hydrogen bonding
(iv) disulfide bridge (covalent)
(v) carboxamide/peptide bond/amide (covalent)
4. The protein should first be hydrolysed by heating with approximately 6 mol dm-3 hydrochloric acid to break it down into its constituent amino acids. A beaker with the bottom covered with about 0.5 cm of a suitable solvent (one that has been previously shown to separate amino acids successfully) should be prepared. A spot of the amino acid mixture is then placed on a line drawn about 1 cm from the bottom of a piece of chromatography paper and samples of known amino acids places at intervals along the same line. The paper is then placed in the beaker so that the solvent can rise up to meet the samples by capillary action. A lid is placed on the beaker to ensure the air inside the beaker is saturated with the vapour of the solvent. When the solvent line has nearly reach the top of the paper, the paper is removed and the position of the solvent front marked. The paper is then hung in a fume cupboard, dried and then sprayed with ninhydrin which colours the spots due to the amino acids. The Rf values of each known amino acid can be calculated and compared with the Rf values obtained for the components of the sample.
5. (a) (i) pH 3.0: H3N+-CH(CH3)-COOH
(ii) pH 6.0: H3N+-CH(CH3)-COO−
(iii) pH 9.0: H2N-CH(CH3)-COO−
(b) The three amino acids are placed in the centre of the polyacrylamide gel and a potential difference applied across it. If a buffer solution with pH 6.3 is used then amino acid B will remain stationary, amino acid C, which has an isoelectric point of pH 7.6 and so contains –NH3+, will move towards the negative electrode, whereas amino acid A, which has an isoelectric point at pH 5.1 will contain -COO−, and so move towards the positive electrode.
6. Altering the pH can mean that the –NH2 and –COOH groups on the end of protein chains can change depending upon isoelectric points. This can alter the shape of the three-dimensional shape (tertiary or quaternary structure) of the enzyme thus affecting the active site, which means the substrate will no longer fit. Large changes in pH may lead to the enzyme becoming broken down completely (denatured).
7. Increasing the temperature of the reaction increases the number of the reacting species that possess the minimum amount of energy (activation energy) necessary for the reaction to occur, hence increasing the rate. However, above a certain temperature the additional energy will cause the enzyme to break down (denature) and lose its catalytic activity.
Download the Answers to Proteins & enzymes questions ![]()

 IB Docs (2) Team
IB Docs (2) Team