Emission & Absorption Spectrum
Line Spectra
- Line spectra is a phenomenon that occurs when excited atoms emit light of certain wavelengths which correspond to different colours
- This comes from differences in discrete energy levels when electrons move between energy levels within an atom
- The emitted light can be observed as a series of coloured lines with dark spaces in between
- These series of coloured lines are called line or atomic spectra
- Each element produces a unique set of spectral lines
- No two elements emit the same set of spectral lines, therefore, elements can be identified by their line spectrum
- There are two types of line spectra: emission spectra and absorption spectra
Emission Spectra
- When an electron transitions from a higher energy level to a lower energy level, this results in the emission of a photon
- Each transition corresponds to a different wavelength of light and this corresponds to a line in the spectrum
- The resulting emission spectrum contains a set of discrete wavelengths, represented by coloured lines on a black background
- Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation:
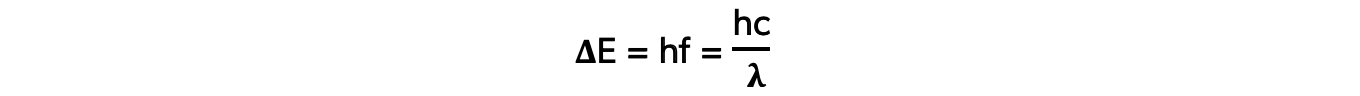
- Where:
- ΔE = change in energy level (J)
- h = Planck’s constant (J s)
- f = frequency of photon (Hz)
- c = the speed of light (m s-1)
- λ = wavelength of the photon (m)
- Therefore, this is evidence to show that electrons in atoms can only transition between discrete energy levels
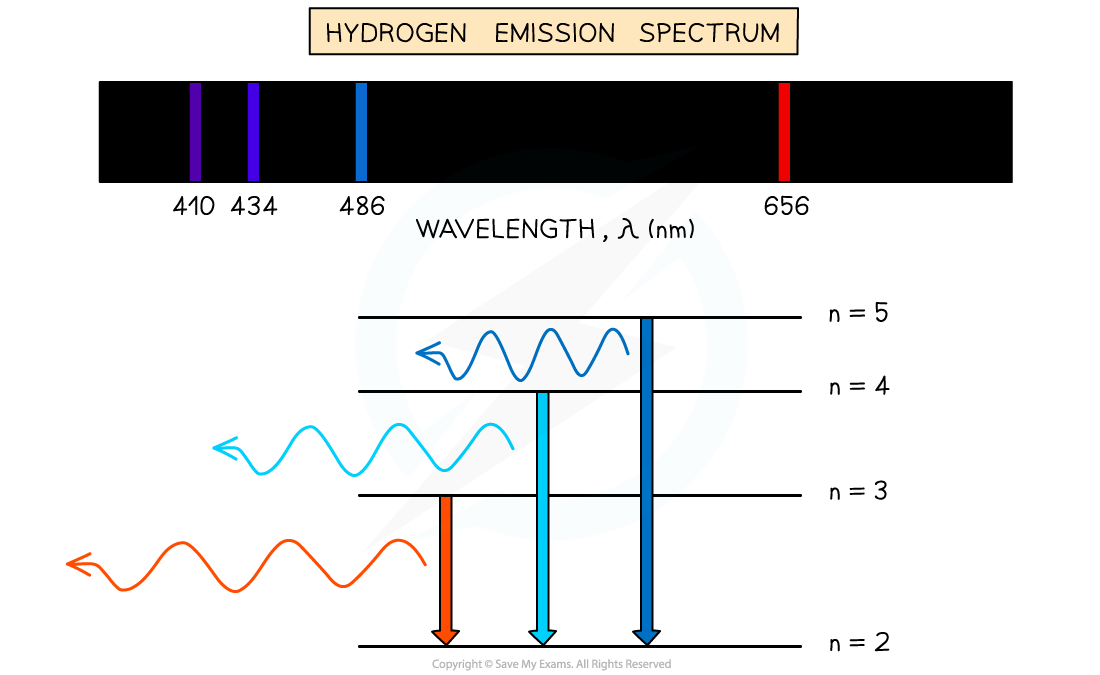
Emission spectrum of Hydrogen gas
Absorption Spectra
- An atom can be raised to an excited state by the absorption of a photon
- When white light passes through a cool, low pressure gas it is found that light of certain wavelengths are missing
- This type of spectrum is called an absorption spectrum
- An absorption spectrum consists of a continuous spectrum containing all the colours with dark lines at certain wavelengths
- These dark lines correspond exactly to the differences in energy levels in an atom
- When these electrons return to lower levels, the photons are emitted in all directions, rather than in the original direction of the white light
- Therefore, some wavelengths appear to be missing
- The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element
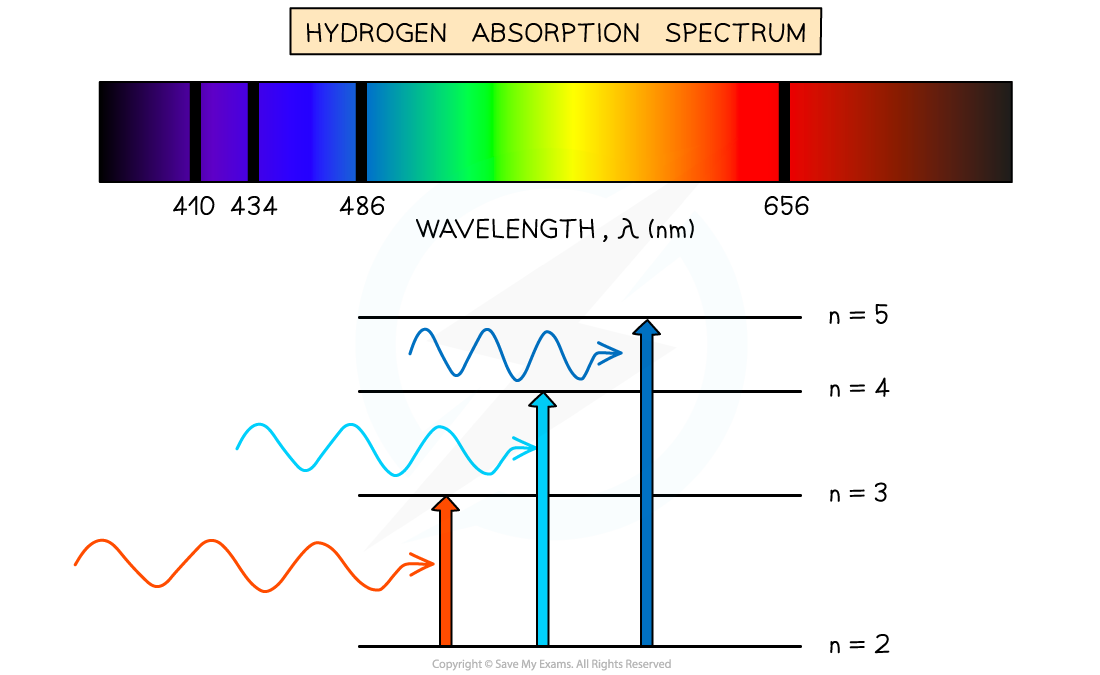
Absorption spectrum of Hydrogen gas
The Hydrogen Spectrum
- A larger version of the hydrogen spectrum from the infrared to the ultraviolet region looks like this

The full hydrogen spectrum
- In the spectrum, we can see sets or families of lines
- Each element will have several series with electrons able to jump between specific energy levels producing specific energy photons
- The Lyman series converges on the ground state for electrons
- The Balmer series converges on the second energy level n = 2
- The Ritz - Paschen converges on the third energy level n = 3 and so on
- The Lyman series photons will have the most energy since they have the shortest wavelength
- The Pfund series photons will have the least energy since they have the longest wavelength
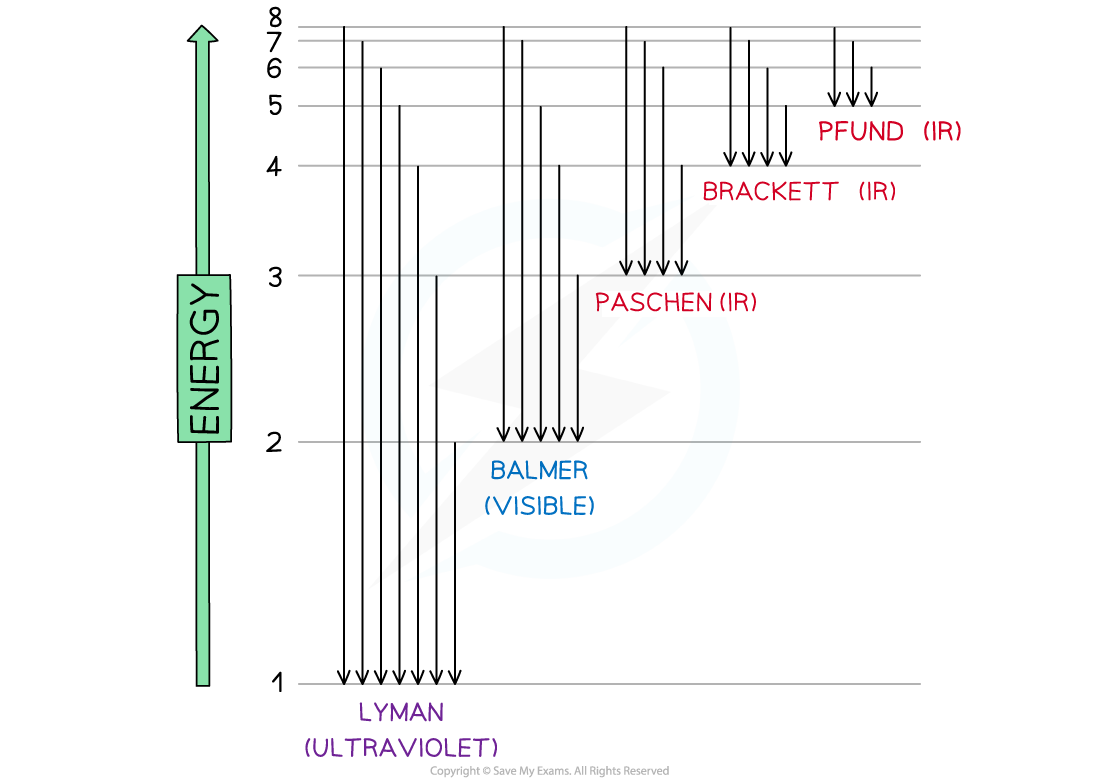
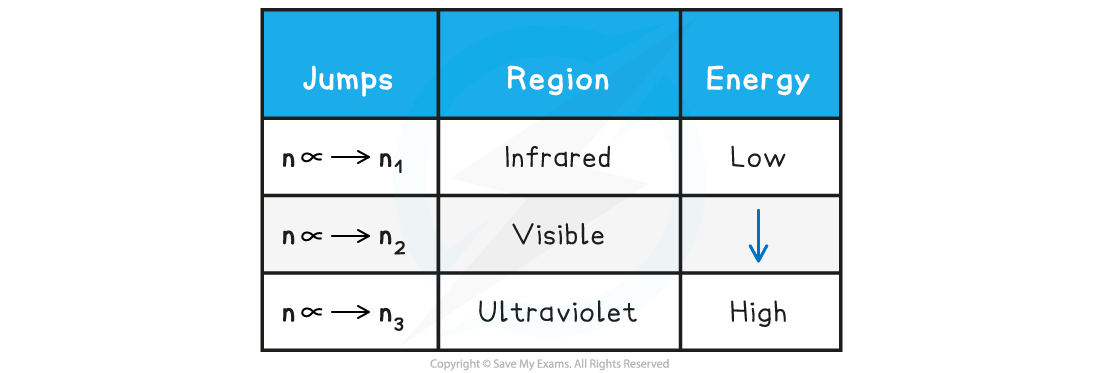
Electron jumps in the hydrogen spectrum
- The finding of these electron jumps helped scientists to understand how electrons work and produce photons of specific wavelength and energy
- The jumps can be summarised as follows:
Electron Jumps & Energy Table