Measuring Half-Life
- Half-life is defined as:
The time taken for the initial number of nuclei to halve for a particular isotope
- This means when a time equal to the half-life has passed, the activity of the sample will also half
- This is because the activity is proportional to the number of undecayed nuclei, A ∝ N
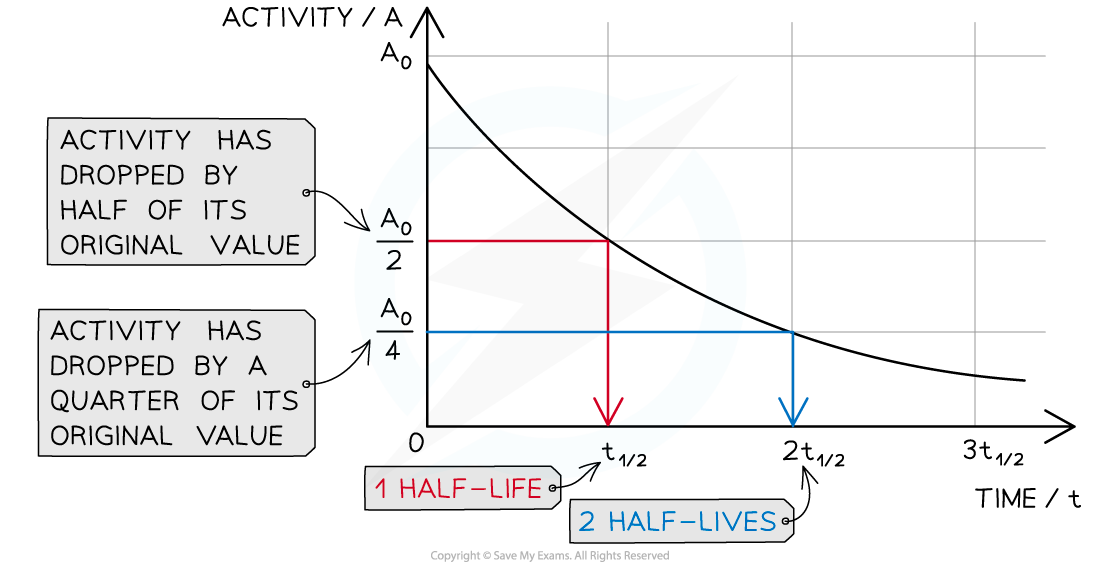
When a time equal to the half-life passes, the activity falls by half, when two half-lives pass, the activity falls by another half (which is a quarter of the initial value)
- To find an expression for half-life, start with the equation for exponential decay:
N = N0 e–λt
- Where:
- N = number of nuclei remaining in a sample
- N0 = the initial number of undecayed nuclei (when t = 0)
- λ = decay constant (s-1)
- t = time interval (s)
- When time t is equal to the half-life t½, the activity N of the sample will be half of its original value, so N = ½ N0
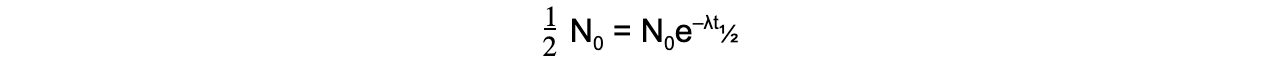
- The formula can then be derived as follows:
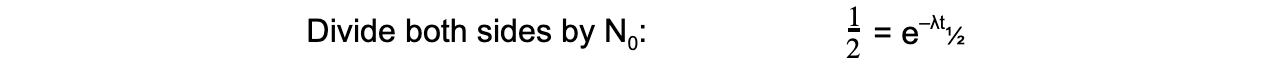


- Therefore, half-life t½ can be calculated using the equation:
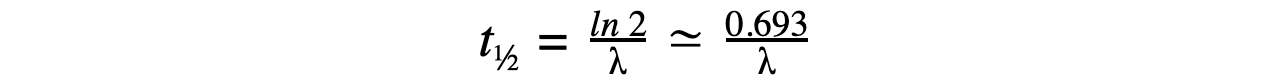
- This equation shows that half-life t½ and the radioactive decay rate constant λ are inversely proportional
- Therefore, the shorter the half-life, the larger the decay constant and the faster the decay
- The half-life of a radioactive substance can be determined from decay curves and log graphs
- Since half-life is the time taken for the initial number of nuclei, or activity, to reduce by half, it can be found by
- Drawing a line to the curve at the point where the activity has dropped to half of its original value
- Drawing a line from the curve to the time axis, this is the half-life
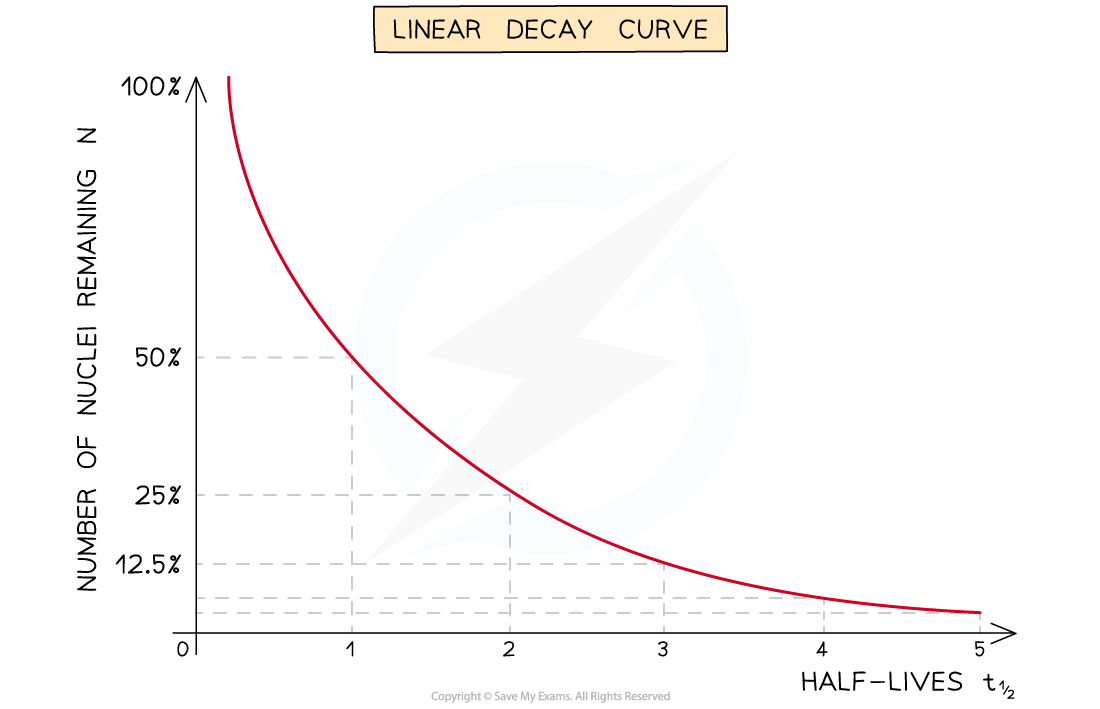
A linear decay curve. This represents the relationship:
Measuring Long Half-Lives
- For nuclides with long half-lives, on the scale of years, this can be measured by:
- Measuring the mass of the nuclide in a pure sample
- Determining the number of atoms N in the sample using N = nNA
- Measuring the total activity A of the sample using the counts collected by a detector
- Determining the decay constant using
- Calculating half-life using
- Note: The sample must be sufficiently large enough in order for a significant number of decays to occur per unit time so that an accurate measure of activity can be made
Measuring Short Half-Lives
- For nuclides with short half-lives, on the scale of seconds, hours or days, this can be measured by:
- Measuring the background count rate in the laboratory (to subtract from each reading)
- Taking readings of the count rate against time until the value equals that of the background count rate (i.e. until all of the sample has decayed)
- Plotting a graph of activity, A, against time, t (as corrected count rate ∝ activity, A)
- Making at least 3 estimates of half-life from the graph and taking a mean
OR
-
- Plotting a graph of ln N against time, t (as corrected count rate ∝ number of nuclei in the sample, N)
- Finding the gradient of this graph, which gives –λ
- Calculating half-life using
- Straight-line graphs tend to be more useful than curves for interpreting data
- Due to the exponential nature of radioactive decay logarithms can be used to achieve a straight line graph
- Take the exponential decay equation for the number of nuclei
N = N0 e–λt
- Taking the natural logs of both sides
ln N = ln (N0) − λt
- In this form, this equation can be compared to the equation of a straight line
y = mx + c
- Where:
- ln (N) is plotted on the y-axis
- t is plotted on the x-axis
- gradient = −λ
- y-intercept = ln (N0)
- Half-lives can be found in a similar way to the decay curve but the intervals will be regular as shown below:
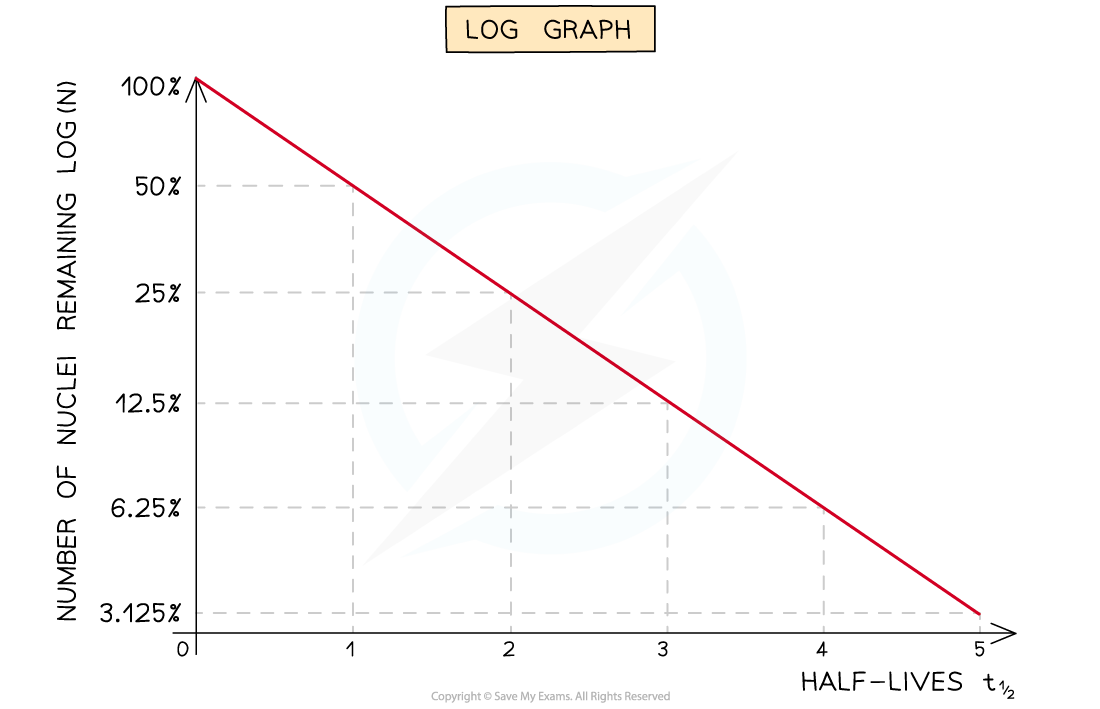
A logarithmic graph. This represents the relationship:
- Note: experimentally, the measurement generally taken is the count rate of the source
- Since count rate ∝ activity ∝ number of nuclei, the graphs will all take the same shapes when plotted against time (or number of half-lives) linearly or logarithmically
Worked Example
Strontium-90 is a radioactive isotope with a half-life of 28.0 years. A sample of Strontium-90 has an activity of 6.4 × 109 Bq.
Part (a)
Step 1: List the known quantities
-
- Half-life, t½ = 28 years
Step 2: Write the equation for half-life
Step 3: Rearrange for λ and calculate
= 0.025 year−1
Part (b)
Step 1: List the known quantities
-
- Decay constant, λ = 0.025 year−1
- Time passed, t = 50 years
Step 2: Write the equation for exponential decay
Step 3: Rearrange for and calculate
= 0.287
-
- Therefore, 28.7% of the sample will remain after 50 years
Worked Example
The radioisotope technetium is used extensively in medicine. The graph below shows how the activity of a sample varies with time.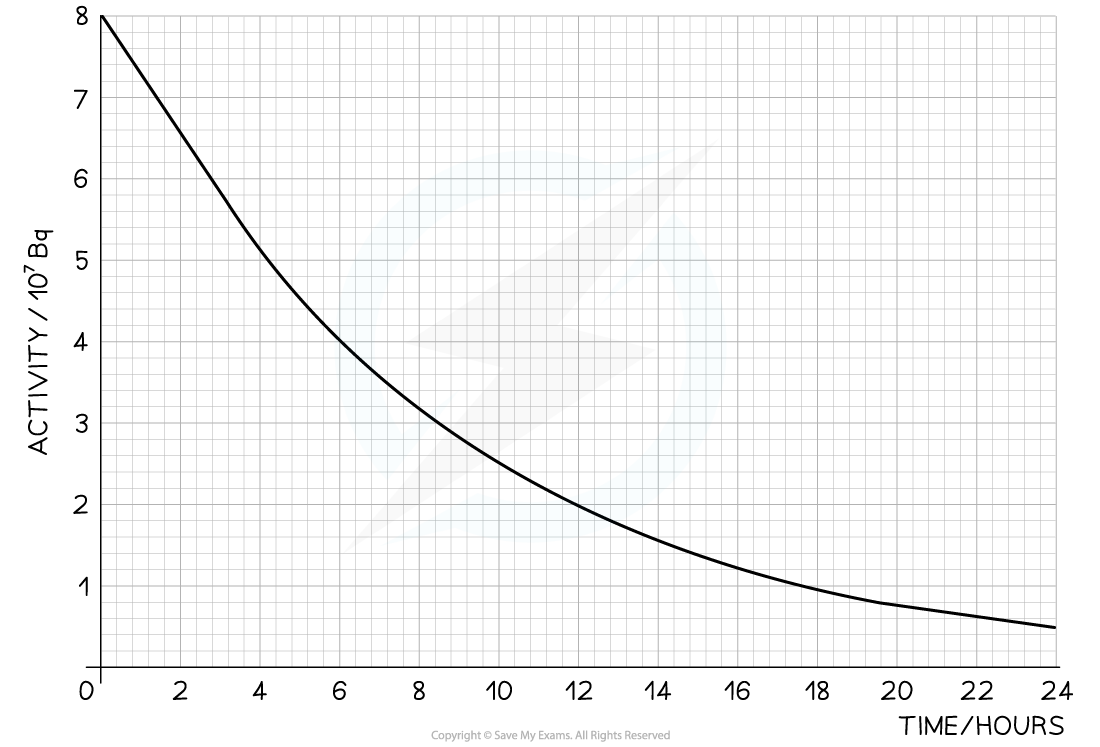
Determine the number of technetium atoms remaining in the sample after 24 hours.
Step 1: Draw lines on the graph to determine the time it takes for technetium to drop to half of its original activity
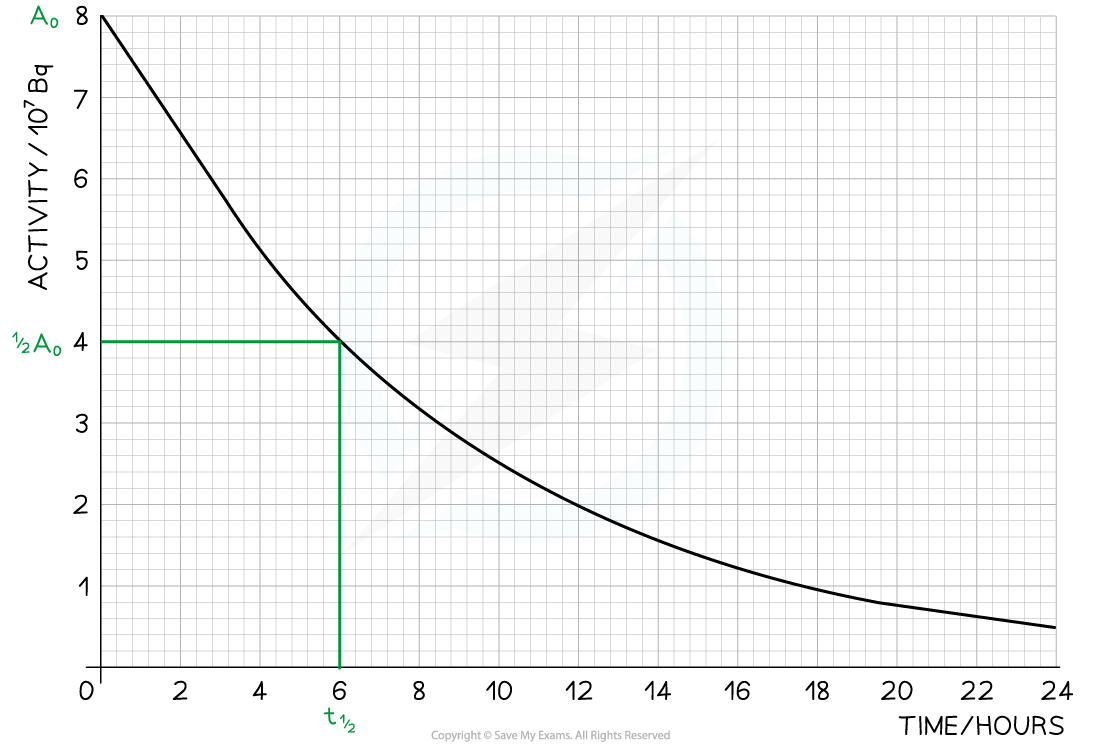
Step 2: Read the half-life from the graph and convert to seconds
-
- t ½ = 6 hours = 6 × 60 × 60 = 21 600 s
Step 3: Write out the half life equation
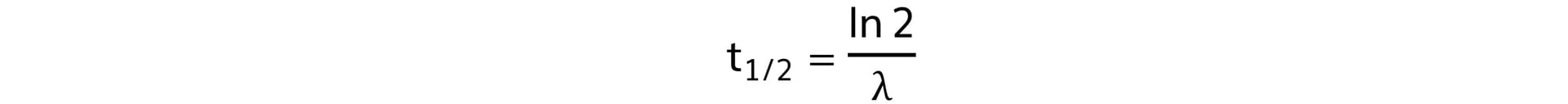
Step 4: Calculate the decay constant
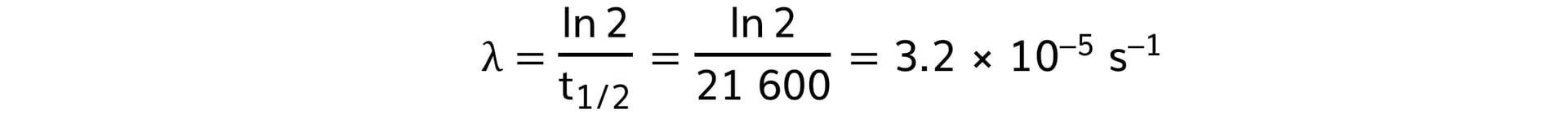
Step 5: Draw lines on the graph to determine the activity after 24 hours
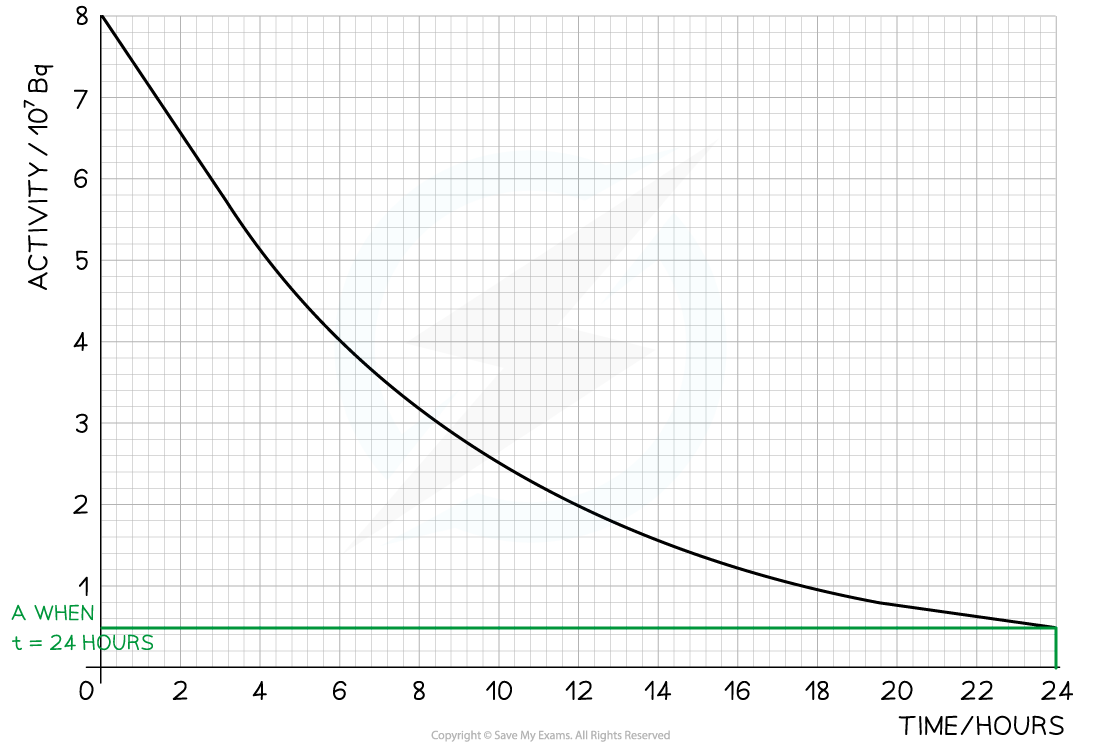
-
- At t = 24 hours, A = 0.5 × 107 Bq
Step 6: Write out the activity equation
A = λN
Step 7: Calculate the number of atoms remaining in the sample

