Deviations from Rutherford Scattering
- Rutherford's alpha scattering experiment originally assumed that the alpha particles are only interacting through electrostatic repulsion
- However, if the energy of the alpha particles exceeds 27.5 - 28 MeV, then they will be close enough to interact with the nucleus via the strong nuclear force
- The Rutherford formula describes this as it states that as the angle of scattering angle increases, the number of alpha particles scattered at that angle sharply decreases
- Where:
- N = number of alpha particles
- θ = angle of scattering
- Rearranging this equation shows that it should be the case that:
- Where:
- k = a constant
- Experimental data bears this out, suggesting that the Rutherford formula is correct
- A number of assumptions are made in deriving this formula, with the main one being that the only force we need to consider is the electric force
- Deviations from Rutherford scattering are evidence of the strong nuclear force
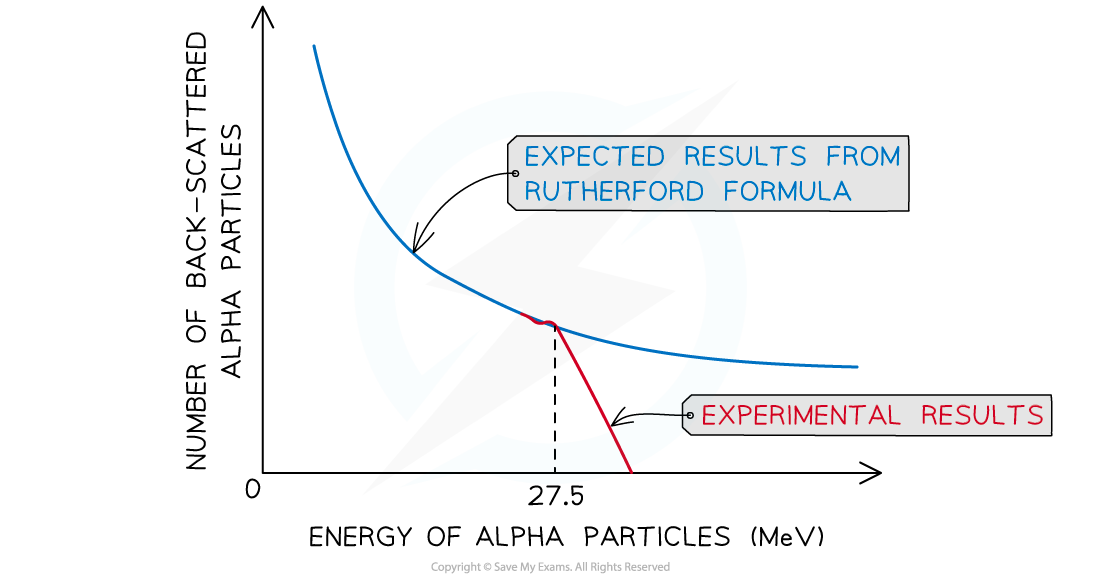
The observed back-scattering from alpha particles strongly deviates from the predicted relationship based only on electromagnetic repulsion at 27.5 MeV
Worked Example
Alpha particles undergo scattering after being fired at a thin gold foil. The gold is then replaced to make a comparison.
Describe the predicted difference in the scattering pattern when the foil is replaced with aluminium foil of the same thickness.
Step 1: Compare the relative charges of the nuclei
-
- Gold has 79 protons, aluminium has 13 protons.
- The electric force between the nuclei is proportional to the charge, since,
-
- Therefore the alpha particle will approach the aluminium nucleus at a much closer distance
Step 2: Consider what causes deviation from Rutherford scattering
-
- Deviations from Rutherford scattering occur when forces apart from the electric force between the nuclei are at play
- At much smaller distances the effect of the strong nuclear force will be felt
Step 3: Deduce the solution
-
- The alpha particles get closer to the aluminium nucleus, at a distance the strong nuclear force begins to act
- More deviation will be seen with aluminium foil than with gold foil
Exam Tip
The greatest deviations from Rutherford scattering occur when the energy of the alpha particles is high and the radius of the target nuclei is small (meaning it has a small nucleon number)
