Greenhouse Gases
- When radiation from the Sun hits the Earth, it is radiated back from the Earth's surface as long-wave radiation
- A greenhouse gas is a gas that absorbs this re-radiated radiation, trapping it in the Earth's atmosphere so that it is not lost to space
- Greenhouse gases in the atmosphere have a similar effect to the glass in a greenhouse, hence the term greenhouse gas
- There are many greenhouse gases, and those that contribute most to the greenhouse effect are:
- Carbon dioxide (CO2)
- Water vapour (H2O)
- These have the most significant impact on the greenhouse effect
- There are other greenhouse gases which have a lesser effect, such as:
- Ozone (O2 and O3)
- Methane
- Nitrous oxides
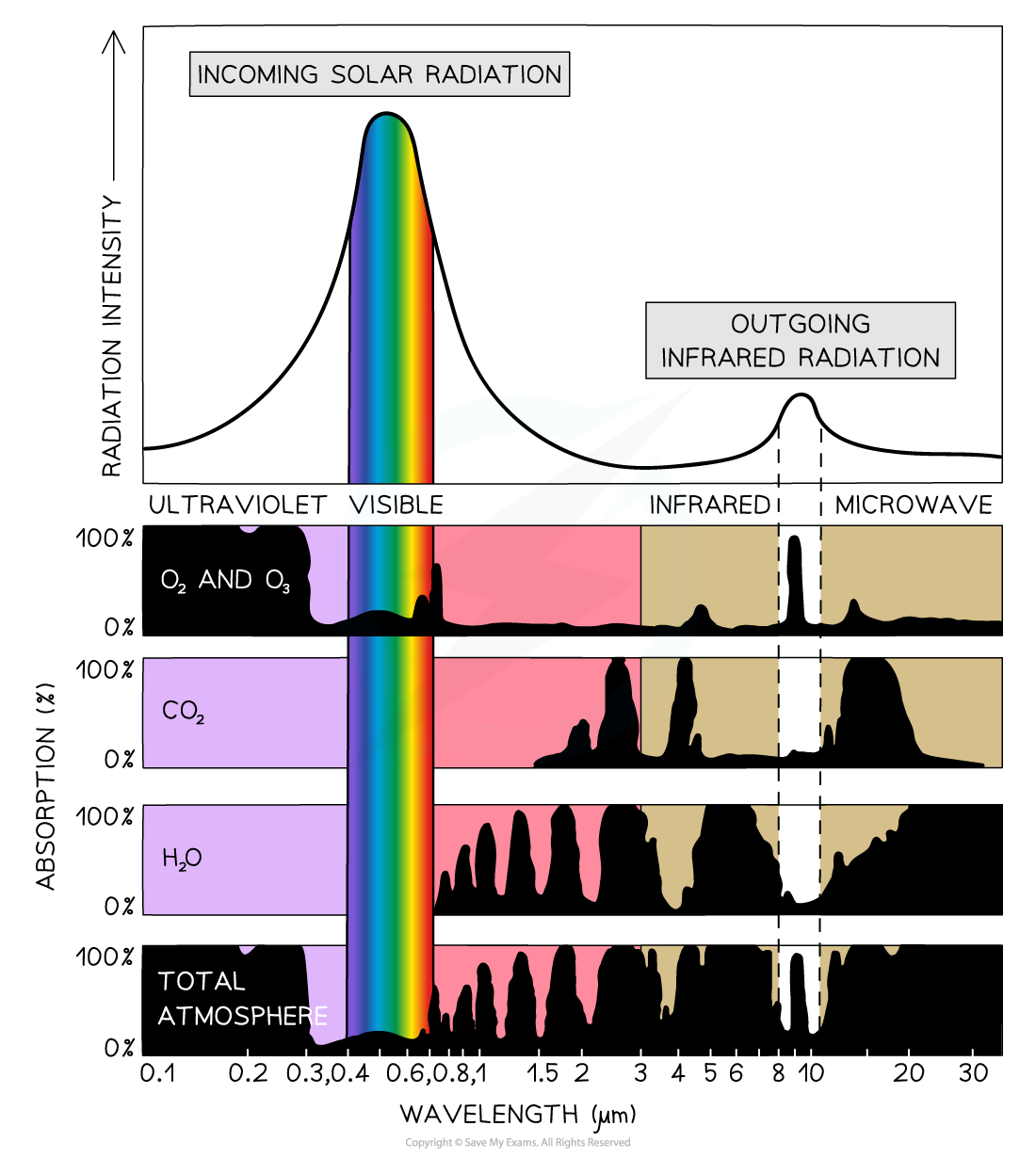
Greenhouse Gas Absorption Spectrum: Ozone absorbs nearly 100% ultraviolet rays, carbon dioxide absorbs radiation with wavelengths between 1.5 - 30 µm and water vapour from 0.8 - 35 µm. Most of the ultraviolet, visible, infrared and microwave radiation is absorbed by the atmosphere. The dark parts show radiation that is absorbed by each type of greenhouse gas. It is the ozone that restricts most of the outgoing infrared radiation from leaving the Earth's atmosphere.
Molecular Mechanisms
- The greenhouse effect occurs due to the particular molecular structure of greenhouse gases
- High-frequency UV light is energetic and able to break bonds within molecules
- Infrared light, on the other hand, causes atoms to vibrate
- The greenhouse gases have a natural frequency that falls in the infrared region
- This means when they absorb infrared light, they begin to resonate, causing the molecules to heat up
- They absorb the infrared radiation and subsequently emit it back towards the Earth’s surface
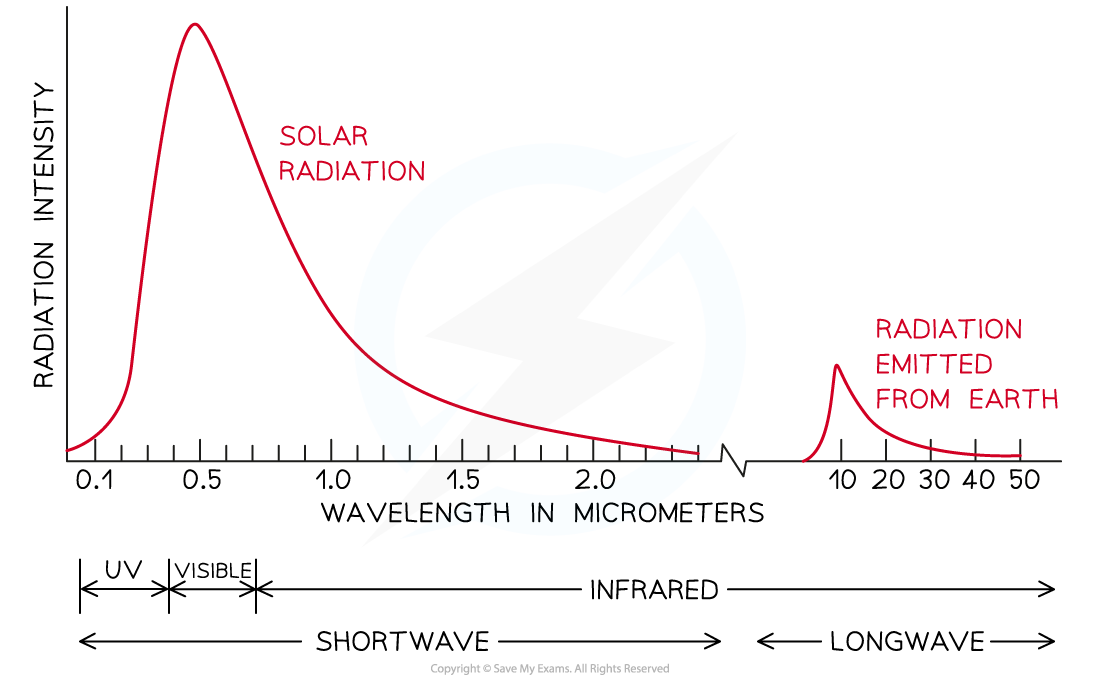
Solar radiation is primarily short-wave, while the radiation that is re-emitted by earth is long-wave radiation
Exam Tip
You may have heard of a separate environmental concern, described as the 'hole in the ozone layer'; this is not something that you need to know about. Ozone is an atmospheric gas that absorbs harmful UV radiation before it reaches earth, but any concerns about ozone depletion have nothing to do with the greenhouse effect. The problem of ozone depletion is one that has improved significantly due to measures taken to reduce certain types of emissions; humans can get it right sometimes!
You do not need to know the specific sources of each type of greenhouse gas – all you need to know is that each greenhouse gas has both natural and man-made origins
The Greenhouse Effect
- While only around 25% of the (primarily short wavelength) solar radiation is absorbed by the atmosphere on its way to Earth, around 80% of the (long wavelength) re-emitted radiation from Earth is absorbed on its way back into the atmosphere
- For example, incoming UV radiation is absorbed by ozone
- Re-emitted infrared radiation is absorbed by greenhouse gases
- This absorbed radiation keeps Earth at a habitable temperature
- However, if there is an imbalance in the chemical composition of the atmosphere, this can lead to fluctuations in the Earth’s mean surface temperature
Process of Global Warming
- Incoming radiation from the Sun predominantly takes the form of ultraviolet and visible radiation
- Visible light is not absorbed by the atmosphere, instead, it is absorbed by the Earth’s surface
- At night, the Earth re-radiates this radiation as infrared
- Some of this radiation is absorbed by the Earth’s atmosphere and some of the radiation is reflected back into space
- The greenhouse gases present in the atmosphere absorb infrared radiation and reflect it back towards the Earth’s surface
- The higher the concentration of greenhouse gases present, the more infrared radiation that remains in the Earth’s surface-atmosphere system
- Therefore, heat energy becomes trapped inside Earth’s atmosphere and accumulates
- This leads to the greenhouse effect and an increase in average mean temperatures on Earth
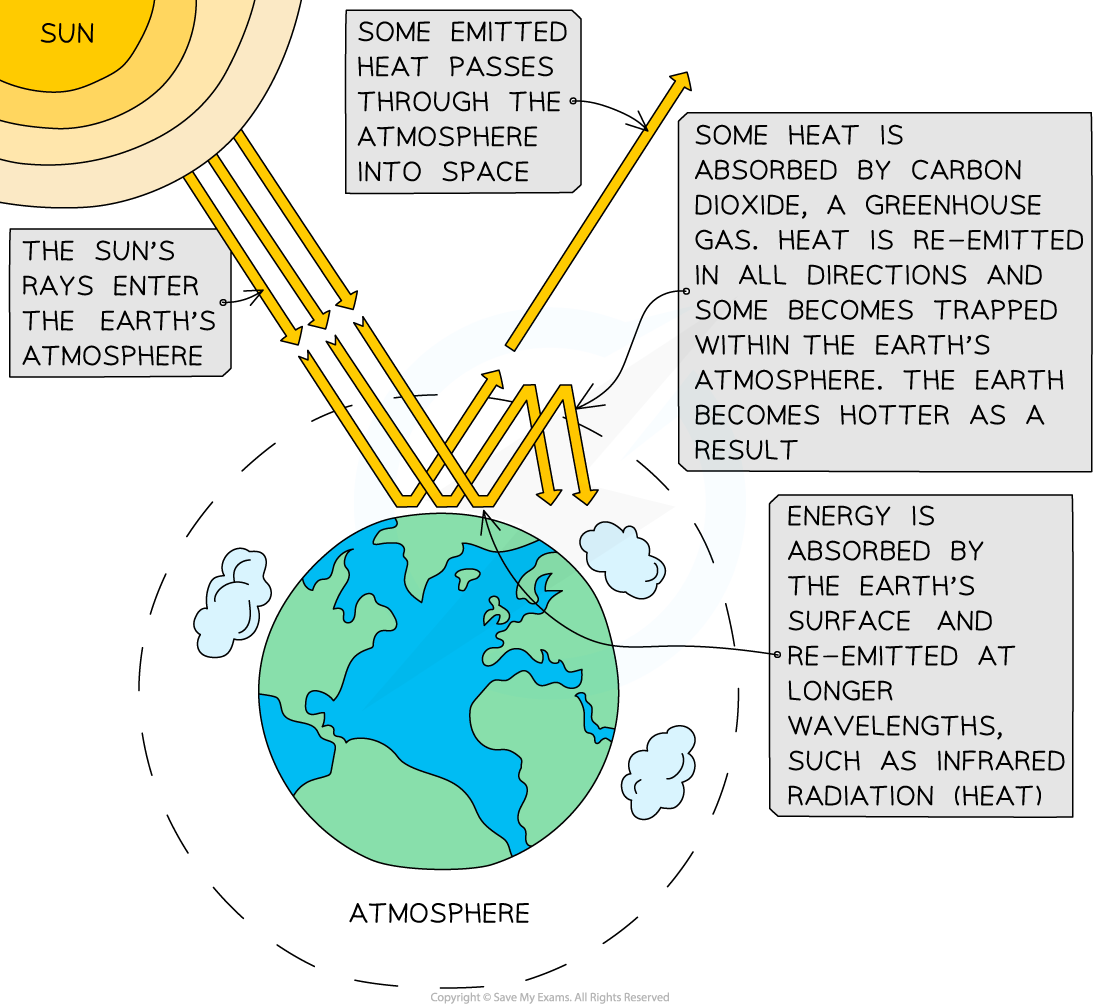
Greenhouse gases absorb the long-wave radiation emitted by Earth, warming the atmosphere
- There are many mechanisms that may increase the rate of global warming
- As the temperature of the Earth increases:
- Ice and snow will melt – this will lead to a decrease in albedo and hence, an increased rate of heat absorption
- The solubility of carbon dioxide in the sea will decrease – this will lead to an increase in atmospheric carbon dioxide concentration
- Surface water will evaporate – this will lead to an increase in atmospheric water vapour concentration
- As a result, small increases in global temperature can lead to large runaway chain reactions which lead to catastrophic climate changes, such as
- Significant rises in sea level due to the melting of ice
- Extreme weather such as heatwaves and heavy floods
