Nuclear Scattering
- Electrons accelerated to close to the speed of light have wave-like properties such as the ability to diffract and have a de Broglie wavelength equal to:

- Where:
- h = Planck's constant
- m = mass of an electron (kg)
- v = speed of the electrons (m s−1)
- When beams of neutrons or electrons are directed at a nucleus they will diffract around it
- The pattern formed by this diffraction has a predictable minimum which forms at an angle θ to the original direction according to the equation
- The diffraction pattern forms a central bright spot with dimmer concentric circles around it
- From this pattern, a graph of intensity against diffraction angle can be used to find the diffraction angle of the first minimum
- The graph of intensity against angle obtained through electron diffraction is as follows:

The first minimum of the intensity-angle graph can be used to determine nuclear radius
- Using this, the size of the atomic nucleus, R, can be determined using:
- Where:
- θ = angle of the first minimum (degrees)
- λ = de Broglie wavelength (m)
- R = radius of the nucleus (m)
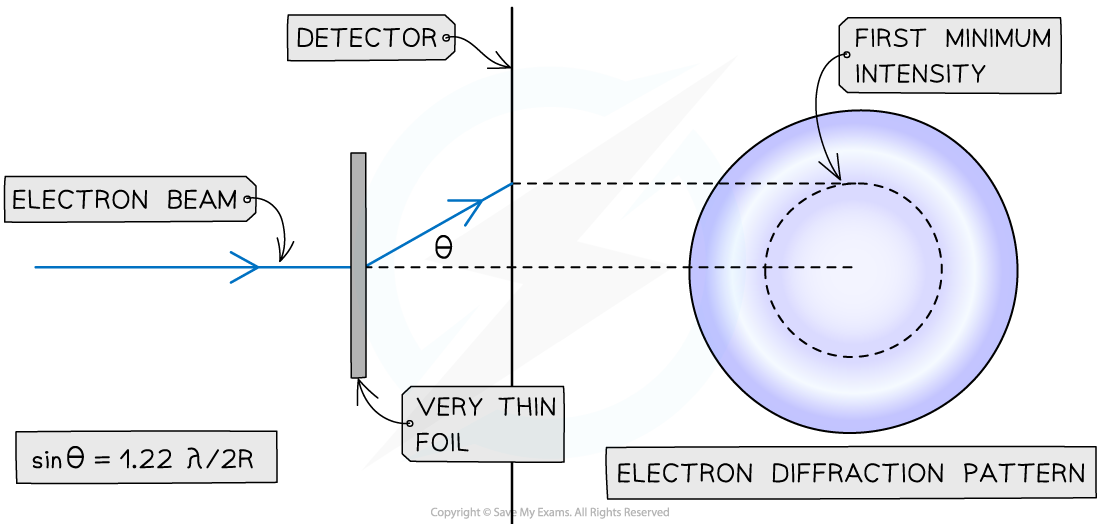
Geometry of electron diffraction
Worked Example
The graph shows how the relative intensity of the scattered electrons varies with angle due to diffraction by the oxygen-16 nuclei. The angle is measured from the original direction of the beam.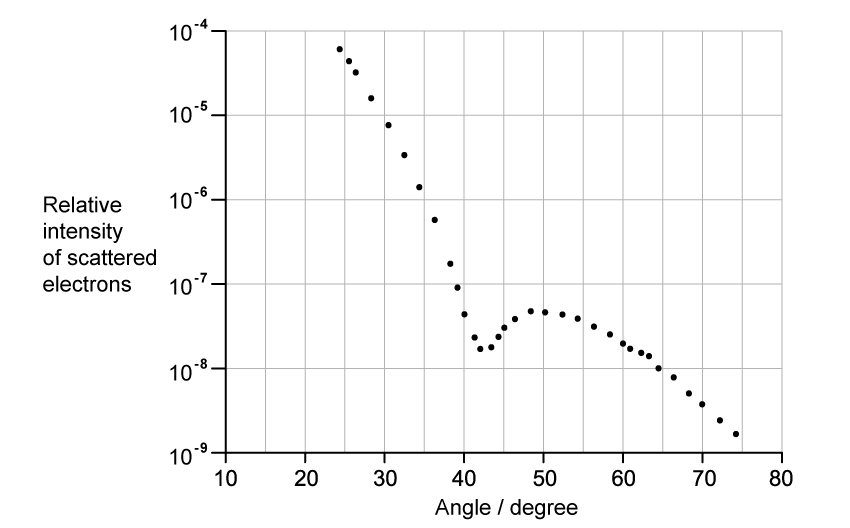
The de Broglie wavelength λ of each electron in the beam is 4.22 × 10−15 m.
Calculate the radius of an oxygen-16 nucleus using information from the graph.
Step 1: Identify the first minimum from the graph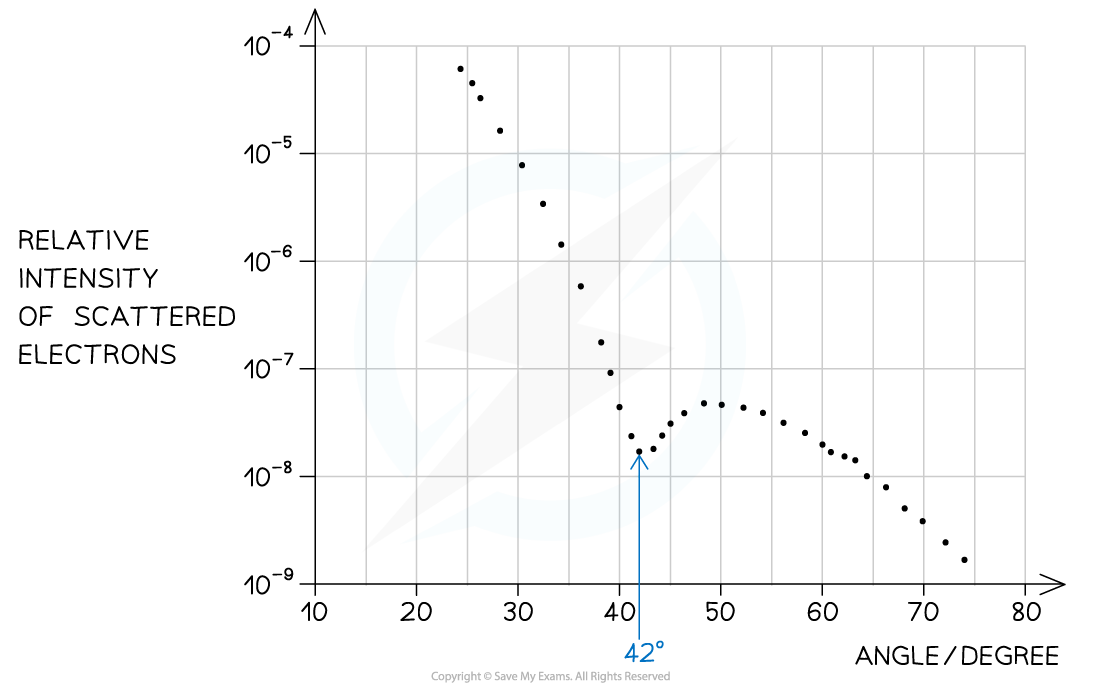
-
- Angle of first minimum, θ = 42°
Step 2: Write out the equation relating the angle, wavelength, and nuclear radius
Step 3: Calculate the nuclear radius, R
= 3.15 × 10−15 m
Exam Tip
In the data booklet, you may notice the relation between angle and diameter appears a couple of times - in the Resolution topic, you are given the full equation for calculating the Rayleigh criterion
However, in this topic, you are given the approximated version
This means you only need to use that version in exam questions about nuclear scattering, omitting the factor of 1.22 is acceptable and likely expected unless asked!
