Atomic Mass Unit
- The unified atomic mass unit u is roughly equal to the mass of one proton or neutron:
- 1u = 1.66 × 10−27 kg
- It is sometimes abbreviated to a.m.u
- This value will be given on your data booklet in the exam
- The a.m.u is commonly used in nuclear physics to express the mass of subatomic particles.
- It is defined as:
1 u is a twelfth of the mass of a (neutral) atom of carbon-12
Worked Example
This question concerns nuclear mass:
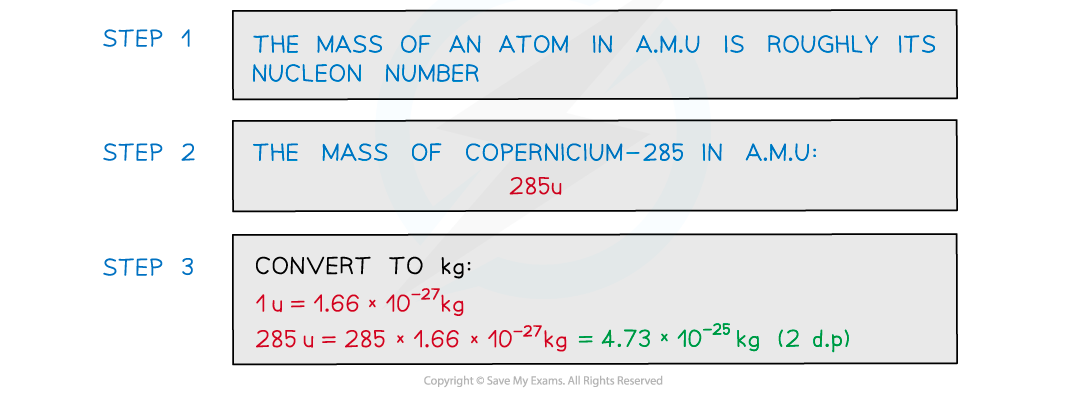
a) Estimate the mass of the nucleus of element copernicium-285 in kg.
Give your answer to 2 decimal places.
b) Using the values for proton and neutron mass given in the data booklet, determine the total mass of all components for a nucleus of element copernicium-285 in kg.
Give your answer to 5 decimal places.
Part (a)
Part (b)
Step 1: Identify the components of copernicium-285
-
- Since the element is copernicium which is the 112th element, this atom must have 112 protons
- Therefore, it will have:
285 – 112 = 173 neutrons in its nucleus
Step 2: Determine the amount of atomic mass units for these components
-
- The proton's mass can be found by multiplying the number of them by their mass in amu which is given in the data booklet:
112 × 1.007276 = 112.814912 u
-
- The neutron's mass similarly can be found using their relevant amu mass:
173 × 1.008665 = 174.499045 u
Step 3: Combine the amu mass of the protons and neutrons
-
- This combination is a mass of:
112.814912 + 174.499045 = 287.313957 u
Step 4: Convert the amount of mass from amu to kg
-
- The conversion is done using the value:
- 1 u = 1.66 × 10−27 kg
- The conversion is done using the value:
287.313957 × 1.66 × 10−27 = 4.7694116862 × 10-25 kg
Step 5: State the final answer to 5 decimal places
-
- The final answer for the total mass of all components of nucleus of element copernicium-285 is approximately:
4.76941 × 10-25 kg
Exam Tip
In most of the IB DP Physics course, significant figures would be expected to be rounded to 2 or 3 digits as the situation or problem deems appropriate. In topic 7, Atomic, Nuclear, and Particle physics, often you will need to continue to answer to 6 significant figures. This is because the level of precision can and will change an answer to a nuclear physics question for different levels of rounding.
