Photons
- In classical wave theory, electromagnetic (EM) radiation is assumed to behave as a wave
- This is demonstrated by the fact EM radiation exhibits phenomena such as diffraction and interference
- However, experiments from the last century, such as discovering the photoelectric effect and atomic line spectra, can only be explained if the EM radiation is thought of as behaving as particles
- These experiments have formed the basis of quantum theory, which will be explored in detail in this section
Photons
- Photons are fundamental particles which make up all forms of electromagnetic radiation
- A photon is defined as
A massless ''packet'' or a ''quantum'' of electromagnetic energy

A wave packet (photon)
- It is also defined as:
A massless particle that possesses an energy equal to E = hf
- This means is that the energy of a photon is not transferred continuously, but as discrete packets of energy
- In other words, each photon carries a specific amount of energy, and transfers this energy all in one go, rather than supplying a consistent amount of energy
- The energy of a photon can be calculated using the formula:
- Substituting in from the wave equation, energy can also be equal to:
- Where:
- E = energy of the photon (J)
- h = Planck's constant (J s)
- c = the speed of light (m s-1)
- f = frequency in Hertz (Hz)
- λ = wavelength (m)
- This equation tells us:
- The higher the frequency of EM radiation, the higher the energy of the photon
- The energy of a photon is inversely proportional to the wavelength
- A long-wavelength photon of light has a lower energy than a shorter-wavelength photon
Worked Example
Light of wavelength 490 nm is incident normally on a surface, as shown in the diagram.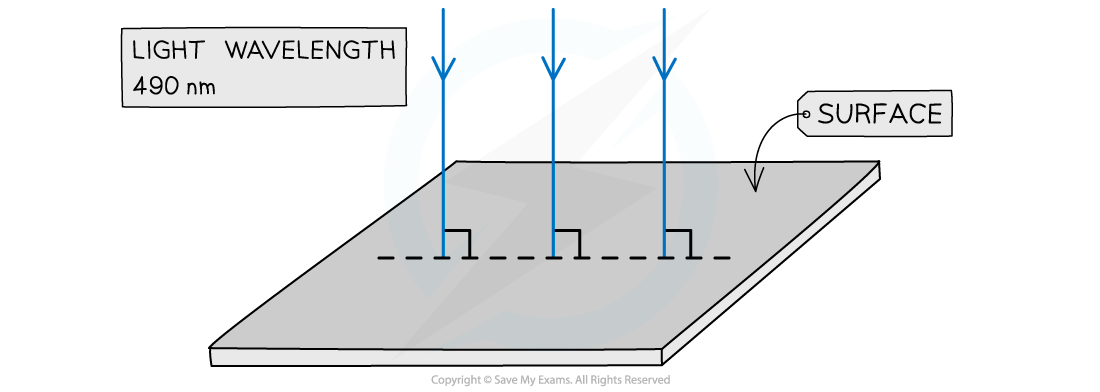
The power of the light is 3.6 mW. The light is completely absorbed by the surface.
Calculate the number of photons incident on the surface in 2.0 s.
Step 1: Write down the known quantities
-
- Wavelength, λ = 490 nm = 490 × 10−9 m
- Power, P = 3.6 mW = 3.6 × 10−3 W
- Time, t = 2.0 s
Step 2: Write the equations for wave speed and photon energy
Step 3: Calculate the energy of one photon
Step 4: Calculate the number of photons hitting the surface every second
Step 5: Calculate the number of photons that hit the surface in 2 s
Photon Momentum
- Einstein showed that a photon travelling in a vacuum has momentum, despite it having no mass
- The momentum (p) of a photon is related to its energy (E) by the equation:
- Where
- c = the speed of light
- p = the momentum of the photon (kg m s-1)
- E = the energy of the photon (J)
Worked Example
Assuming that all the photons are absorbed by the plate, calculate the momentum of the photon, and the pressure exerted by the laser beam on the metal plate.
Step 1: Write down the known quantities
-
- Power, P = 5.0 mW = 5.0 × 10−3 W
- Frequency, f = 5.6 × 1014 Hz
- Cross-sectional area, A = 8.0 × 10−6 m2
Step 2: Write the equations for photon energy and momentum
Step 3: Calculate the photon momentum
Step 4: Calculate the number of photons incident on the plate every second
Step 5: Calculate the force exerted on the plate in a 1.0 s time interval
Step 6: Calculate the pressure
Exam Tip
The values of Planck’s constant and the speed of light will always be given to you in an exam, however, it helps to memorise them to speed up calculation questions!
