The Ionic Product of Water
pH of water
- An equilibrium exists in water where few water molecules dissociate into proton and hydroxide ions
H2O(l) ⇌ H+(aq) + OH-(aq)
- The equilibrium constant for this reaction is:
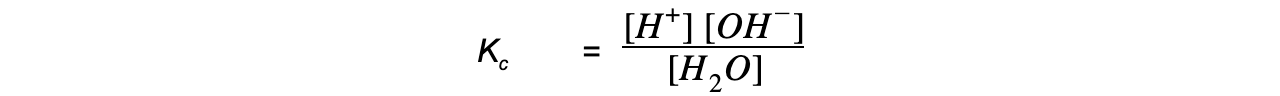
Kc x [H2O] = [H+] [OH-]
- Since the concentration the H+ and OH- ions is very small, the concentration of water is considered to be a constant, such that the expression can be rewritten as:
Kw = [H+] [OH-]
Where Kw (ionic product of water) = Kc x [H2O]
= 10-14 mol2 dm-6 at 298K
- The product of the two ion concentrations is always 10-14 mol2 dm-6
- This makes it straightforward to see the relationship between the two concentrations and the nature of the solution:
[H+] & [OH-] Table

Worked Example
What is the pH of a solution of potassium hydroxide, KOH(aq) of concentration 1.0 × 10−3 mol dm−3 ?Kw = 1.0 × 10−14 mol2 dm-6
A. 3
B. 4
C. 10
D. 11
Answer:
The correct option is D.
- Since Kw = [H+] [OH-] , rearranging gives [H+] = Kw ÷ [OH-]
- The concentration of [H+] is (1.0 × 10−14) ÷ (1.0 × 10−3) = 1.0 × 10−11 mol dm−3
- So the pH = 11
