Kinetic Theory
- The kinetic theory developed in the 18th century out of a need to explain how it is that gases exert pressure inside a container
- Theories about gas particles and movement were extended to include all states of matter
- The kinetic theory of matter accounts for the properties of solids, liquids, and gases in terms of the interactions of particles and their relative energies
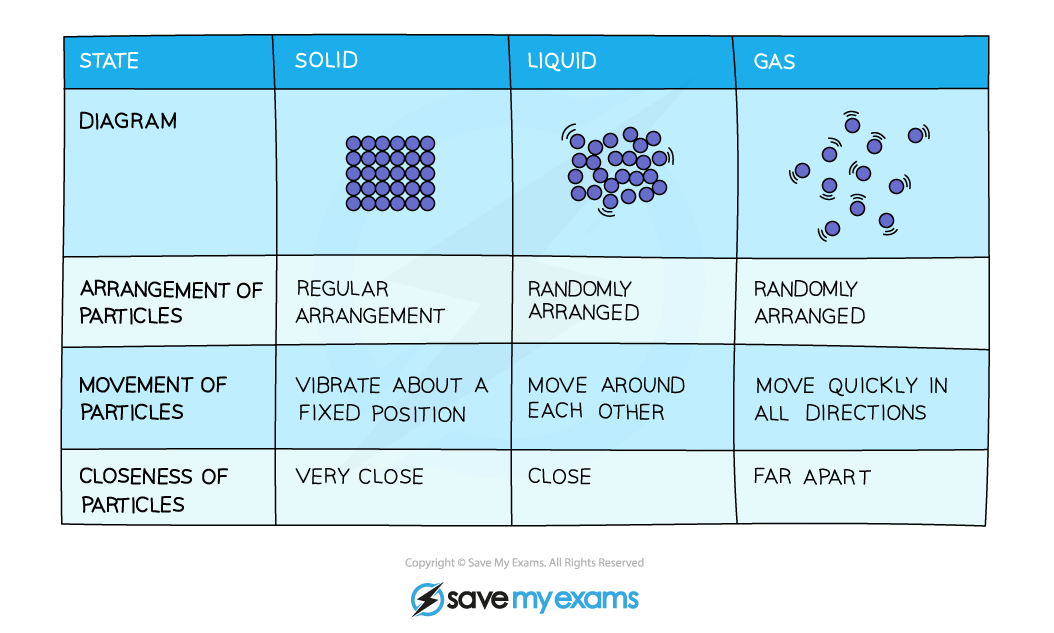
Summary Table of the Kinetic Theory
- The average kinetic energy of the particles is directly proportional to the temperature of the system in Kelvin
- Kinetic energy refers to the energy associated with movement or motion. It is determined by the mass and velocity of the substance according to the relationship:
KE = ½mv2
- As the kinetic energy of the particles at the same temperature is equal, this means there is an inverse relationship between mass and velocity
- This is why substances with a lower mass diffuse more quickly than those with greater mass at the same temperature
Collision Theory
Collision Theory
- When reactants come together the kinetic energy they possess means their particles will collide and some of these collisions will result in chemical bonds being broken and some new bonds being formed
- The rate of a chemical reaction depends on four factors:
- collision frequency
- collision energy
- activation energy
- collision geometry
Collision frequency
- If a chemical reaction is to take place between two particles, they must first collide
- The number of collisions between particles per unit time in a system is known as the collision frequency
- The collision frequency of a given system can be altered by changing the concentration of the reactants, by changing the total pressure, by changing the temperature or by changing the size of the reacting particles
Collision energy
- Not all collisions result in a chemical reaction
- Most collisions just result in the colliding particles bouncing off each other
- Collisions which do not result in a reaction are known as unsuccessful collisions
- Unsuccessful collisions happen when the colliding species do not have enough energy to break the necessary bonds
- If they do not have sufficient energy, the collision will not result in a chemical reaction
- If they have sufficient energy, they will react, and the collision will be successful
- The combined energy of the colliding particles is known as the collision energy
Collision energy is the combined energy of two colliding particles
Activation Energy
- The minimum energy the colliding particles need in order to react is known as the activation energy
- If the collision energy of the colliding particles is less than the activation energy, the collision will be unsuccessful
- If the collision energy is equal to or greater than the activation energy, the collision will be successful, and a reaction will take place
- The activation energy can be changed by the addition of a catalyst

Molecules with the activation energy lead to successful collisions
Collision Geometry
- Particles have to have the right orientation when they collide for the reaction to be successful
- This is particularly the case with large molecules with complex shapes
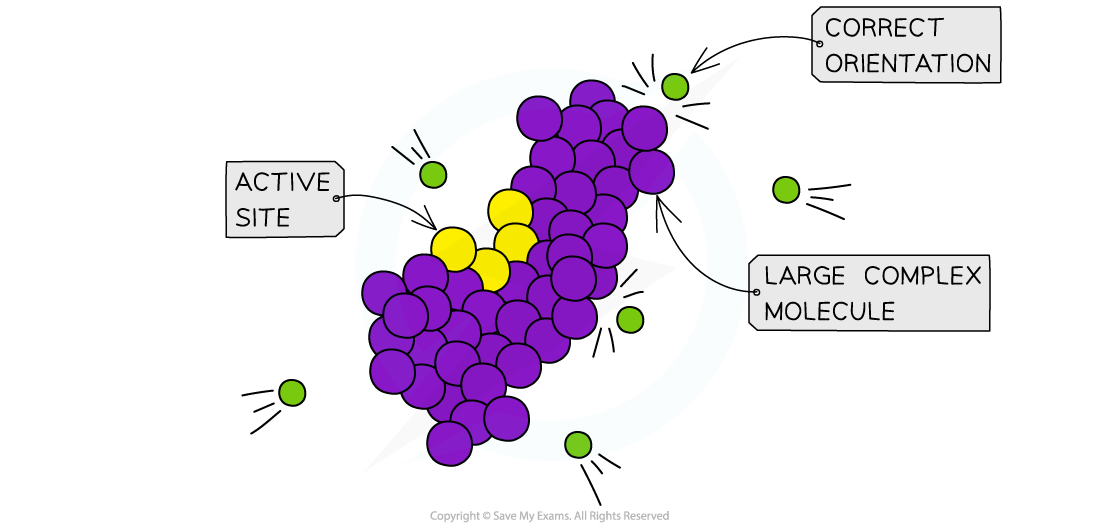
Orientation becomes increasingly important in large complex biomolecules such as proteins and carbohydrates where active sites (reactive part of the molecule) can only be accessed in one orientation
- Most collisions do not result in reaction because they do not reach the activation energy rather than not having the correct collision geometry
Summary Table of Collision Theory Factors