Lewis Theory of Acids & Bases
- A more general definition of acids and bases was given by G.N. Lewis who defined them as:
- A Lewis acid is an electron pair acceptor
- A Lewis base is an electron pair donor

General mechanism for Lewis acids and bases
- This enabled a wider range of substances to be classed as acids or bases
- This can be shown in the following examples in which a hydroxide ion, OH-, and ammonia, NH3, donate a pair of electrons to a hydrogen ion
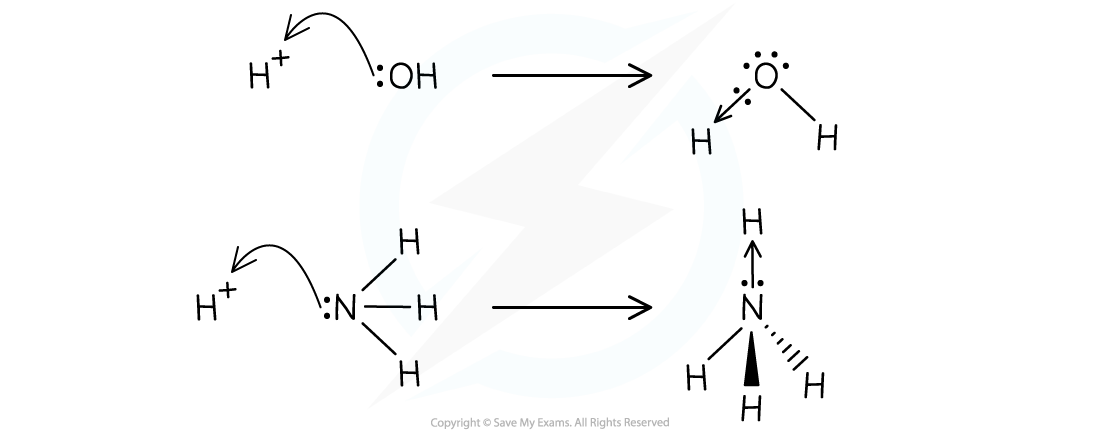
The OH- ion and ammonia act as Lewis bases in both examples by donating an electron pair
Brønsted-Lowry Acids and Bases
- A Brønsted-Lowry acid is a species that can donate a proton
- For example, hydrogen chloride (HCl) is a Brønsted-Lowry acid as it can lose a proton to form a hydrogen (H+) and chloride (Cl–) ion
HCl (aq) → H+ (aq) + Cl– (aq)
- A Brønsted-Lowry base is a species that can accept a proton
- For example, a hydroxide (OH–) ion is a Brønsted-Lowry base as it can accept a proton to form water
OH– (aq) + H+ (aq) → H2O (l)
Weak acids dissociating- In an equilibrium reaction, the products are formed at the same rate as the reactants are used
- This means that at equilibrium, both reactants and products are present in the solution
- For example, ethanoic acid (CH3COOH) is a weak acid that partially dissociates in solution
- When equilibrium is established there are CH3COOH, H2O, CH3COO– and H3O+ ions present in the solution
Lewis Acid, Lewis Base, Brønsted-Lowry acid or Brønsted-Lowry base
- A point to consider when thinking about Lewis acids and bases as well as Brønsted-Lowry acids and bases is the donating and accepting of protons
- Brønsted-Lowry acid and base theory restricts the acids to proton donors only
- A Lewis acid can accept a pair of electrons and it can also accept a proton
- As a lone pair of electrons present a proton can be accepted, the OH- ion is a good example of this
- To be defined as a Brønsted-Lowry acid or base, a proton must be donated or accepted
- This does not of course occur in every reaction
- For example:
- The lone pair on the nitrogen atom in ammonia, NH3 , can be donated to the boron atom in boron trifluoride, BF3, creating a molecule of NH3BF3
- In this case, neither compound reacts as an Brønsted-Lowry acid or Brønsted-Lowry base as no protons (H+ ions) are being donated or accepted
- Only electron pairs are being donated and accepted

Ammonia donates a lone pair of electrons to form a coordinate bond
- Here boron forms three sp2 hybridised orbitals leaving a vacant 2pz orbital which allows the lone pair on the nitrogen atom to form a dative covalent bond
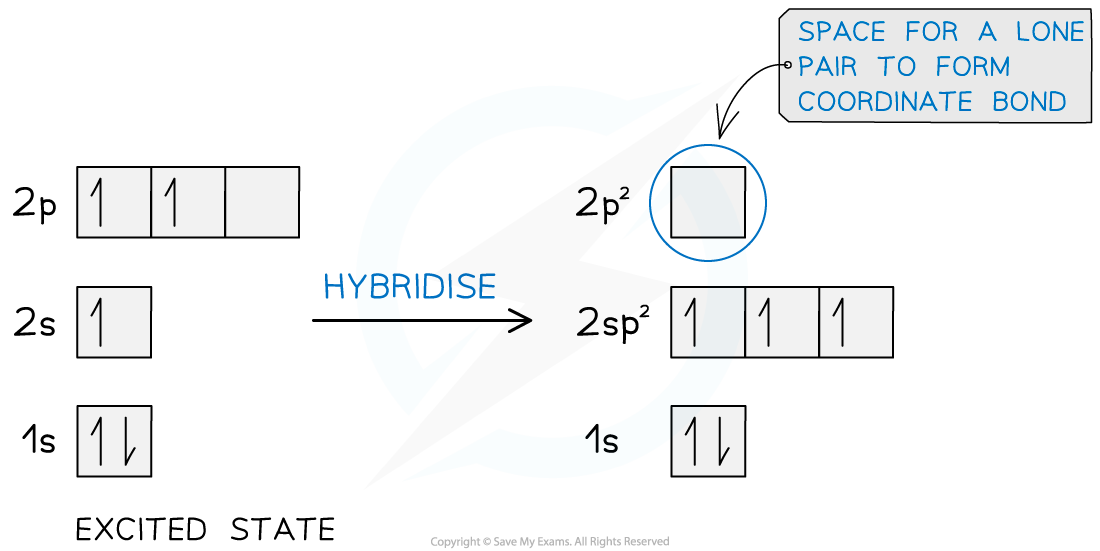
Hybridisation of the boron atom
- The following molecules can behave as either Lewis bases and Brønsted-Lowry acids
- Lewis bases as they can donate an electron pair
- Brønsted-Lowry base as they can accept a proton

Examples of molecules that can behave both as Lewis bases and Brønsted-Lowry base
Identifying Lewis Acids & Bases
- In the case of a complex ion, such as hexaaquacopper(II), the water molecule is acting as a Lewis base and the metal ion is acting as a Lewis acid
- Copper(II), like other transition metals, can form a complex due to a partially occupied d subshell
- Cu2+ (aq) + 6H2O (l) → [Cu(H2O)6]2+ (aq)
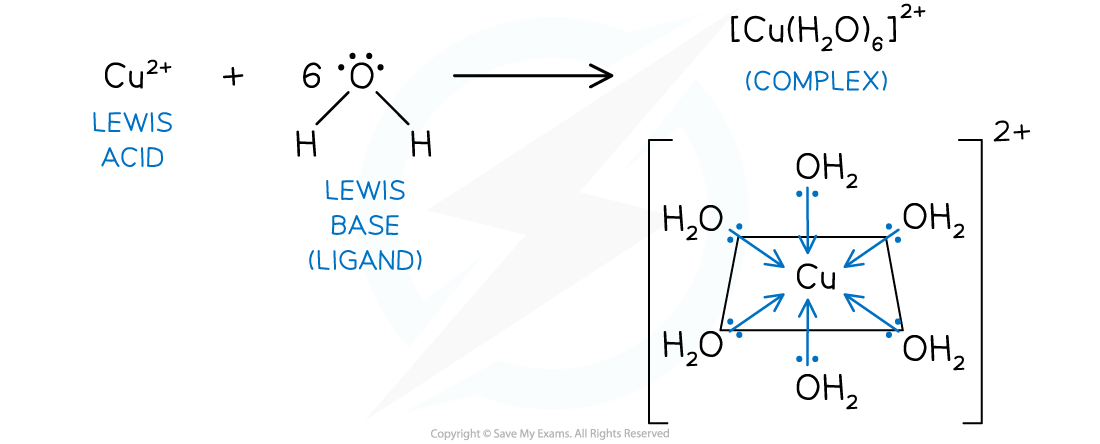
Hexaaquacopper(II) complex
- Given that Lewis acids can donate a pair of electrons, they can be classed as electrophiles
- An electrophile is a electron-deficient species that can accept a lone pair from a nucleophile, in the same way the Cu2+ ion accepts electron pairs from water
- The cyanide ion, -CN , water, H2O , ammonia , NH3 , are examples of Lewis bases and they can also act as nucleophiles
- Nucleophiles are electron rich species with at least on pair of lone electrons
Worked Example
Identify the Lewis acid and Lewis base in the following reaction

Methanoate ion reacting with water
Answer
- The Lewis acid is water, H2O
- The hydrogen in the water molecule is accepting a pair of electrons leaving an OH- ion
- The Lewis base is the methanoate ion, HCOO-
- The lone pair of electrons in the methanoate ion forms a coordinate bond with one of the hydrogens from the water molecule
- The Lewis acid is water, H2O
