Hybrid Orbitals
Hybridisation
- The ground state of the electrons in a carbon atom is 1s22s22p2
- This can be represented using a spin diagram as shown:
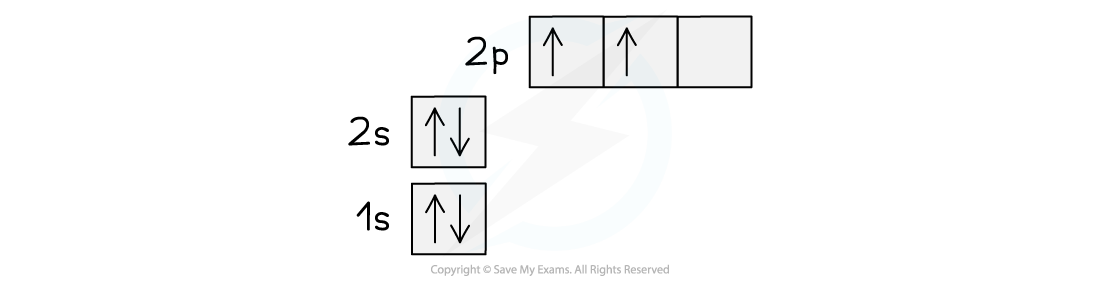
Orbital spin diagram for carbon in the ground state
- This electronic structure would imply that carbon forms two covalent bonds using the unpaired 2p electrons
- Since the 2s electrons are paired there would be no reason for them to be involved in bonding
- However studies of carbon compounds show that carbon typically forms four covalent bonds that are all equal in energy
- This puzzle has been explained using the theory of bond hybridisation
- A half full p-subshell has a slightly lower energy than a partially filled one. The difference in energy between the 2s and 2p subshells is small, so an electron can fairly easily be promoted from the 2s to the 2p giving the new arrangement:
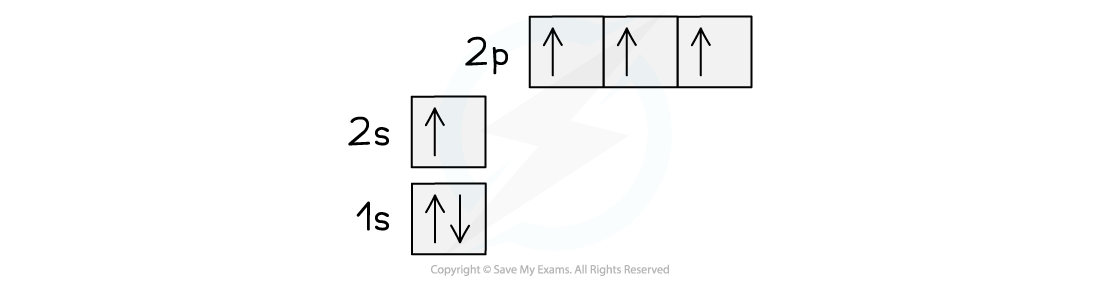
Orbital spin diagram for carbon in the excited state
- The 2s and 2p subshells blend together and form four new hybrid orbitals (called sp3 orbitals, after the merger of an s and 3 p orbitals)
- This would give four unpaired electrons of equal energy, capable of forming four covalent bonds.
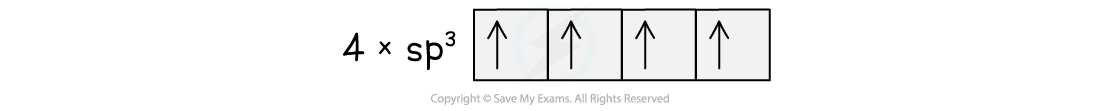
Orbital spin diagram for carbon showing sp3 hybrid orbitals
- The theory of Quantum mechanics shows that the shape of a 1s orbital is spherical and a p orbital is dumbbell or figure-of-eight shaped
- There are three p orbitals all at right angles to each other, known as px, py and pz
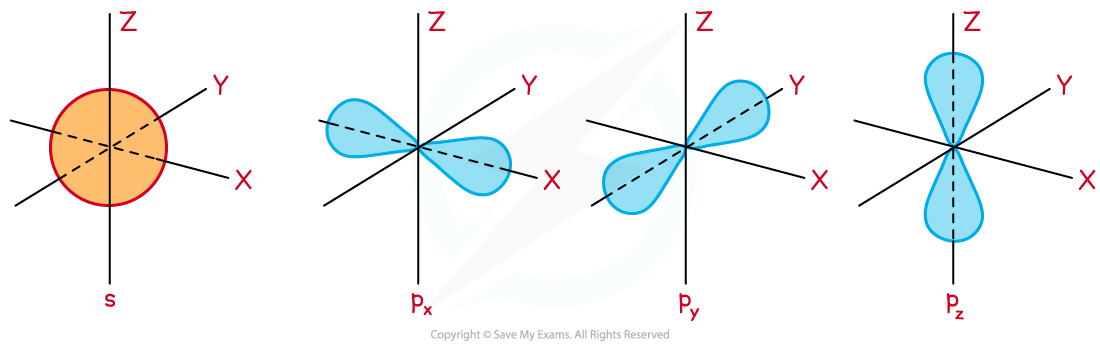
The shape of s and p orbitals
sp3 hybridisation
- Four hybrid orbitals are produced when the 2s and three 2p orbitals blend together
- These hybrids have ¼ s character and ¾ p character so they have a club shape reminiscent of an enlarged p orbital
- The four sp3 hybrid orbitals space themselves out at 109.5o forming a tetrahedron
- This is the resolution of the structure seen when carbon forms single bonds, such as would be found in methane
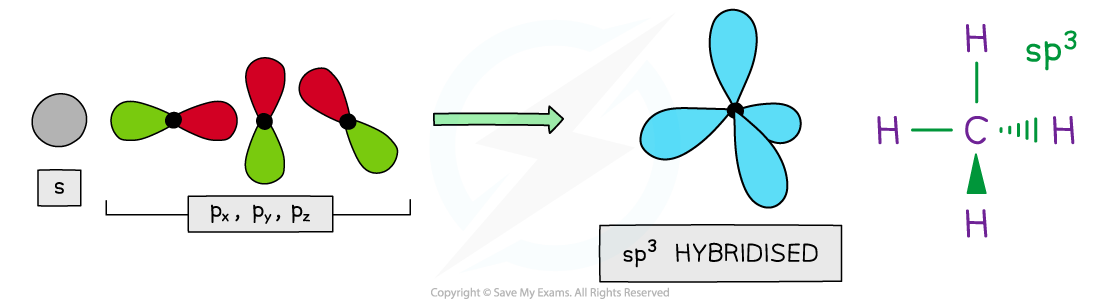
4 x sp3 hybrid orbitals
- The sp3 orbitals merge with the s orbitals in hydrogen forming four equal sigma bonds
- It is not just bonding pairs of electrons that are accommodated in hybrid orbitals - lone pairs can also be present
- The domain geometry of ammonia is tetrahedral due to sp3 hybrid orbitals where three bonding pairs and one lone pair are found
sp2 hybridisation
- Three hybrid orbitals are produced when the 2s and two 2p orbitals blend together
- These hybrids have ⅓ s character and ⅔ p character
- The three sp2 hybrid orbitals space themselves out at 120o forming a trigonal planar geometry
- This is the resolution of the structure seen when carbon forms two single bonds and a double bond with itself in alkenes

3 x sp2 hybrid orbitals
- In the case of carbon, the sp2 orbitals merge with the s orbitals in hydrogen and the sp2 of an adjacent carbon, forming three equal sigma bonds
- The double bond is created by the side-to-side overlap of the unhybridised p-orbitals
- This bonding arrangement can also occur between a double bonded carbon and oxygen so is typically seen in the carbonyl group
sp hybridisation
- Two hybrid orbitals are produced when the 2s and one 2p orbital blend together
- These hybrids have ½ s character and ½ p character
- The two sp hybrid orbitals space themselves out at 180o forming linear geometry
- This is the resolution of the structure seen when carbon forms one single bonds and a triple bond with itself in alkynes
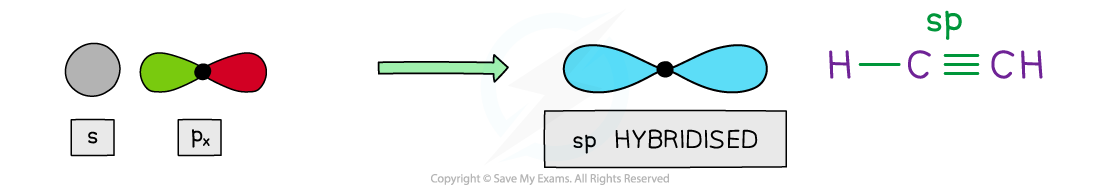
2 x sp hybrid orbitals
- In the case of carbon, the sp orbital merges with the s orbital in hydrogen and the sp of an adjacent carbon, forming two equal sigma bonds
- The triple bond is created by the side-to-side overlap of two pairs of the unhybridised p-orbitals, set at right angles to each other
Identifying Hybridisation
- You can predict the hybridisation present in molecules based on Lewis structures, electron domains, molecular geometries by applying the principles outlines in the previous section
Worked Example
Identify the domain geometry, molecular geometry and hybridisation in the underlined atom ina) CH3COCH3 b) PH3 c) NO2
Answer
a) CH3COCH3

The Lewis structure shows that there are three electron domains around the central carbon, so the domain geometry is trigonal planar. There are two single bonds and one double bond, so the molecular geometry is also trigonal planar, and the carbon must have sp2 hybridisation.
b) PH3

The Lewis structure shows that there are four electron domains around the phosphorus, so the domain geometry is tetrahedral. There are three single single bonds and a lone pair, so the molecular geometry is trigonal pyramid. Four domains means the phosphorus must have sp3 hybridisation.
c) NO2

The Lewis structure shows that there are three electron domains around the nitrogen, so the domain geometry is trigonal planar. There is one single bonds and one double bond, so the molecular geometry is bent linear, and the nitrogen must have sp2 hybridisation
Exam Tip
You may be wondering why the unpaired electron lies on the nitrogen rather than on a oxygen in the Lewis structure for NO2. This is easily demonstrated by considering the formal charges and electronegativity. The preferred Lewis structure has negative charges located on the most electronegative atoms.
FC (N) = V – ½B – N = (5) – ½(6) – 1 = +1
FC (single bonded O) = (6) – ½(2) – 6 = -1
FC (double bonded O) = (6) – ½(4) – 4 = 0
Oxygen and nitrogen have electronegativity of 3.4 and 3.0, respectively (Table 8 in the Data booklet), so placing the electron on the nitrogen means it has a positive FC instead of the oxygen.