Electron Orbitals
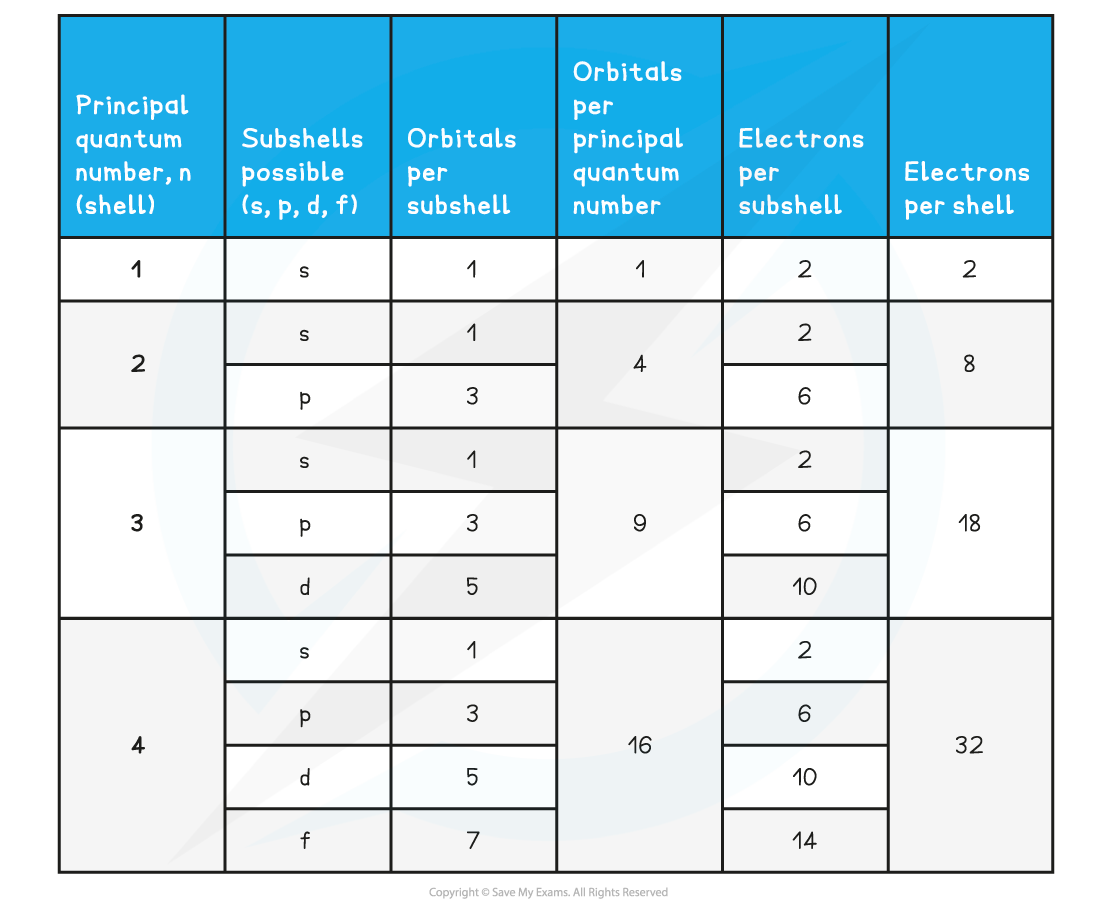
- Each shell can be divided further into subshells, labelled s, p, d and f
- Each subshell can hold a specific number of orbitals:
- s subshell : 1 orbital
- p subshell : 3 orbitals labelled px, py and pz
- d subshell : 5 orbitals
- f subshell : 7 orbitals
- Each orbital can hold a maximum number of 2 electrons so the maximum number of electrons in each subshell are as follows:
- s : 1 x 2 = total of 2 electrons
- p : 3 x 2 = total of 6 electrons
- d : 5 x 2 = total of 10 electrons
- f : 7 x 2 = total of 14 electrons
- In the ground state, orbitals in the same subshell have the same energy and are said to be degenerate, so the energy of a px orbital is the same as a py orbital

Shells are divided into subshells which are further divided into orbitals
Summary of the Arrangement of Electrons in Atoms Table
The s & p Orbitals
s orbitals
- The s orbitals are spherical in shape
- The size of the s orbitals increases with increasing shell number
- E.g. the s orbital of the third quantum shell (n = 3) is bigger than the s orbital of the first quantum shell (n = 1)

The s orbitals become larger with increasing principal quantum number
p orbitals
- The p orbitals are dumbbell-shaped
- Every shell has three p orbitals except for the first one (n = 1)
- The p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another
- The lobes of the p orbitals become larger and longer with increasing shell number

The p orbitals become larger and longer with increasing principal quantum number
