Moles, Particles & Masses
- Since atoms are so small, any sensible laboratory quantity of substance must contain a huge number of atoms
- Such numbers are not convenient to work with, so using moles is a better unit to deal with the sort of quantities of substance normally being measured
- When we need to know the number of particles of a substance, we usually count the number of moles
- The number of moles or particles can be calculated easily using a formula triangle
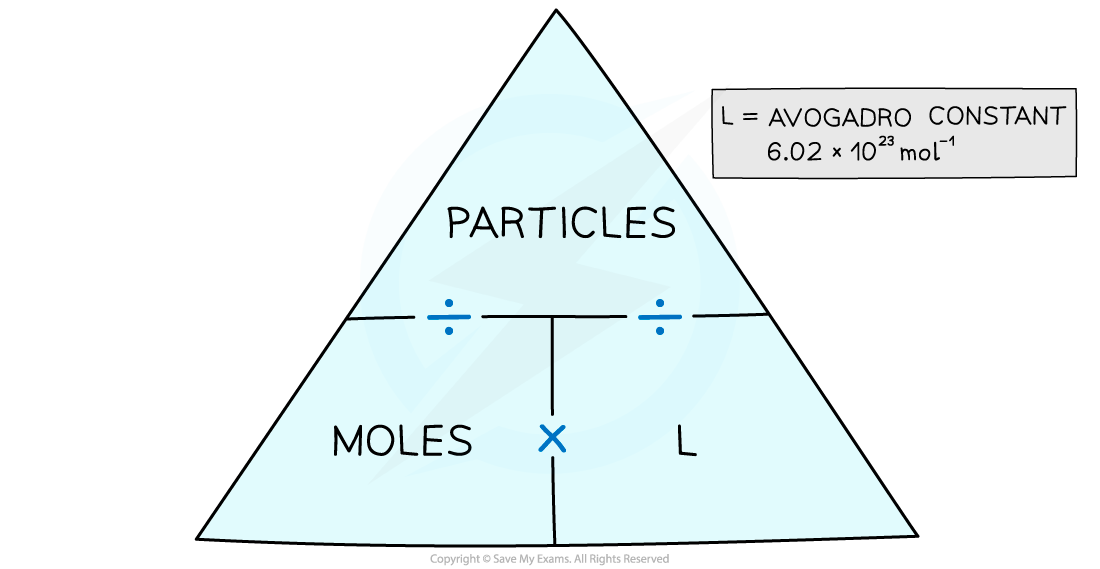
The moles and particles formula triangle – cover with your finger the one you want to find out and follow the directions in the triangle
Worked Example
How many hydrogen atoms are in 0.010 moles of CH3CHO?
Answer:
- There are 4 H atoms in 1 molecule of CH3CHO
- So, there are 0.040 moles of H atoms in 0.010 moles of CH3CHO
- The number of H atoms is the amount in moles x L
- This comes to 0.040 x (6.02 x 1023) = 2.4 x 1022 atoms
Worked Example
How many moles of hydrogen atoms are in 3.612 x 1023 molecules of H2O2?
Answer:
- In 3.612 x 1023 molecules of H2O2 there are 2 x (3.612 x 1023) atoms of H
- So, there are 7.224 x 1023 atoms of H
- The number of moles of H atoms is the number of particles ÷ L
- This comes to 7.224 x 1023 ÷ (6.02 x 1023) = 1.20 moles of H atoms
Moles and Mass
- We count in moles by weighing the mass of substances
- The number of moles can be calculated by using a formula triangle
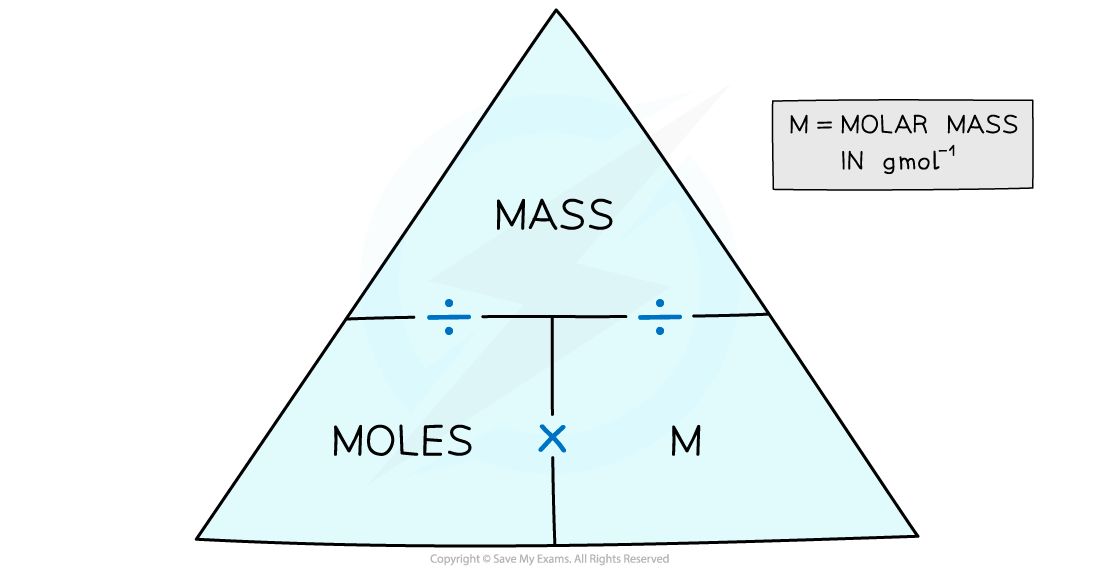
The moles and mass formula triangle – cover with your finger the one you want to find out and follow the directions in the triangle
Worked Example
What is the mass of 0.250 moles of zinc?
Answer:
- From the periodic table the relative atomic mass of Zn is 65.38
- So, the molar mass is 65.38 g mol-1
- The mass is calculated by moles x molar mass
- This comes to 0.250 mol x 65.38 g mol-1 = 16.3 g
Worked Example
How many moles are in 2.64 g of sucrose, C12H11O22 (Mr = 342.3)?
Answer:
- The molar mass of sucrose is 342.3 g mol-1
- The number of moles is found by mass ÷ molar mass
- This comes to 2.64 g ÷ 342.3 g mol-1 = 7.71 x 10-3 mol
Exam Tip
Always show your workings in calculations as its easier to check for errors and you may pick up credit if you get the final answer wrong.
