Giant Covalent Structures
Covalent lattices
- Covalent bonds are bonds between nonmetals in which electrons are shared between the atoms
- In some cases, it is not possible to satisfy the bonding capacity of a substance in the form of a molecule; the bonds between atoms continue indefinitely, and a large lattice is formed. There are no individual molecules and covalent bonding exists between all adjacent atoms
- Such substances are called giant covalent substances, and the most important examples are C and SiO2
- Graphite, diamond, buckminsterfullerene and graphene are allotropes of carbon
Diamond
- Diamond is a giant lattice of carbon atoms
- Each carbon is covalently bonded to four others in a tetrahedral arrangement with a bond angle of 109.5o
- The result is a giant lattice with strong bonds in all directions
- Diamond is the hardest substance known
- For this reason it is used in drills and glass-cutting tools
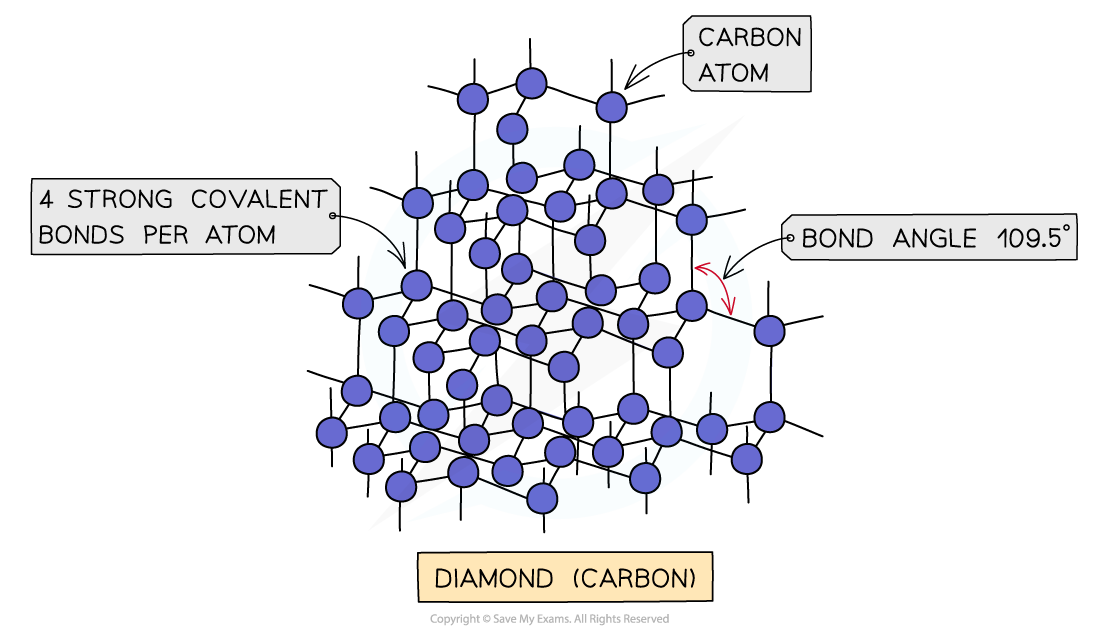
The structure of diamond
Graphite
- In graphite, each carbon atom is bonded to three others in a layered structure
- The layers are made of hexagons with a bond angle of 120o
- The spare electron is delocalised and occupies the space in between the layers
- All atoms in the same layer are held together by strong covalent bonds, and the different layers are held together by weak intermolecular forces
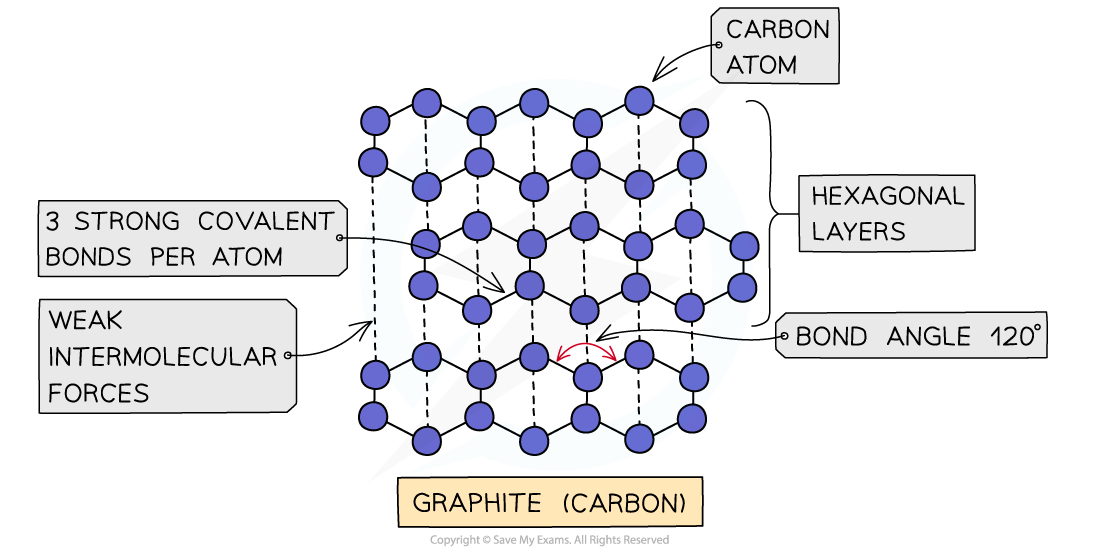
The structure of graphite
Buckminsterfullerene
- Buckminsterfullerene is one type of fullerene, named after Buckminster Fuller, the American architect who designed domes like the Epcot Centre in Florida
- It contains 60 carbon atoms, each of which is bonded to three others by single covalent bonds
- The fourth electron is delocalised so the electrons can migrate throughout the structure making the buckyball a semi-conductor
- It has exactly the same shape as a soccer ball, hence the nickname the football molecule

The structure of buckminsterfullerene
Graphene
- Some substances contain an infinite lattice of covalently bonded atoms in two dimensions only to form layers. Graphene is an example
- Graphene is made of a single layer of carbon atoms that are bonded together in a repeating pattern of hexagons
- Graphene is one million times thinner than paper; so thin that it is actually considered two dimensional

The structure of graphene
Silicon(IV)oxide
- Silicon(IV)oxide is also known as silicon dioxide, but you will be more familiar with it as the white stuff on beaches!
- Silicon(IV)oxide adopts the same structure as diamond - a giant structure made of tetrahedral units all bonded by strong covalent bonds
- Each silicon is shared by four oxygens and each oxygen is shared by two silicons
- This gives an empirical formula of SiO2
![]()
The structure of silicon dioxide
Properties of Giant Structures
- Different types of structure and bonding have different effects on the physical properties of substances such as their melting and boiling points, electrical conductivity and solubility
Covalent bonding & giant covalent lattice structures
- Giant covalent lattices have very high melting and boiling points
- These compounds have a large number of covalent bonds linking the whole structure
- A lot of energy is required to break the lattice
- The compounds can be hard or soft
- Graphite is soft as the forces between the carbon layers are weak
- Diamond and silicon(IV) oxide are hard as it is difficult to break their 3D network of strong covalent bonds
- Graphene is strong, flexible and transparent which it makes it potentially a very useful material
- Most compounds are insoluble with water
- Most compounds do not conduct electricity however some do
- Graphite has delocalised electrons between the carbon layers which can move along the layers when a voltage is applied
- Graphene is an excellent conductors of electricity due to the delocalised electrons
- Buckminsterfullerene is a semi-conductor
- Diamond and silicon(IV) oxide do not conduct electricity as all four outer electrons on every carbon atom is involved in a covalent bond so there are no free electrons available
Characteristics of Giant Covalent Structures Table

Exam Tip
Although buckminsterfullerene is included in this section it is not classified as a giant structure as it has a fixed formula, C60
