Complex Ions
- Transition element ions can form complexes which consist of a central metal ion and ligands
- A ligand is a molecule or ion that forms a co-ordinate bond with a transition metal by donating a pair of electrons to the bond
- This is also the definition of a Lewis base
- This means ligands have a negative charge or a lone pair of electrons capable of being donated
- This definition may seem familiar: a ligand is the same as a nucleophile
- Different ligands can form different numbers of dative bonds to the central metal ion in a complex
- Some ligands can form one co-ordinate bond to the central metal ion
- Other ligands can form two co-ordinate bonds, and some can form multiple dative bonds
- Co-ordination number is number of co-ordinate bonds to the central metal atom or ion
Common Ligands
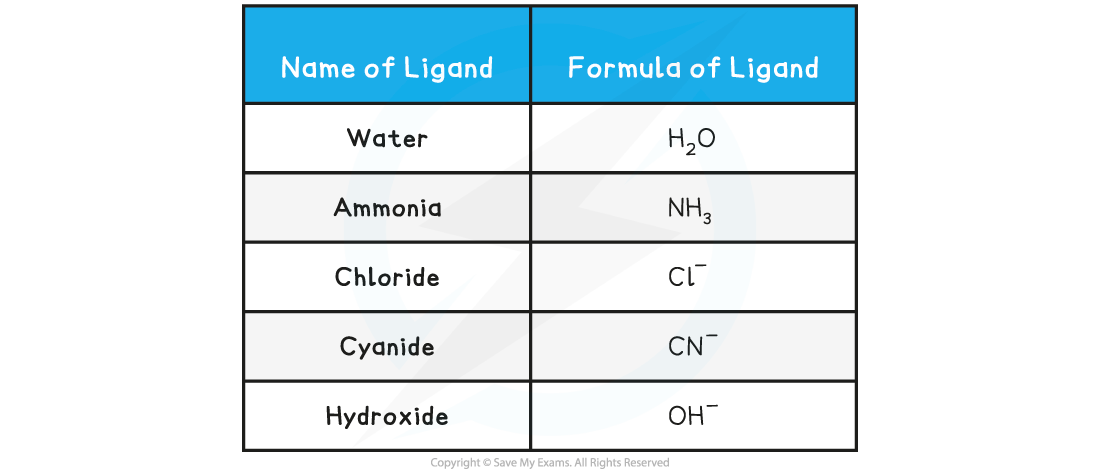
- Water molecules frequently act as ligands. Each water molecule makes a single bond with the metal ion using one of the lone pairs on the oxygen atom
- The lone pair is donated to the partially filled d-subshell of the transition metal ion
Table showing examples of common monodentate ligands
Representing complex ions
- Square brackets are used to group together the ligands and metal ion in a representation of the geometrical arrangement
- The overall charge on the complex ion is the sum of the oxidation states of all the species present
- If the ligands are neutral then the overall charge will be the same as the oxidation state of the metal ion

Examples of complexes with monodentate ligands
Co-ordination number
- The coordination number is the number of coordinate bonds to the metal ions
- This number can be the same as the number of ligands if they are monodentate, but it could be different if bi- or multidentate ligands are present
Naming complexes
- Complexes are named in the following way
- If the overall ion is a cation then the nomenclature is:
Prefix for number of ligands/ligand name/element/oxidation number
- The prefixes are the same ones used in organic chemistry: di, tetra, hexa for 2, 4 & 6 respectively (3 & 5 are rarely encountered except in mixed ligand complexes)
- If the overall ion is an anion, the name of element is modified to have the name ending 'ate' and sometimes Latin word stems are used
- Using the examples in the illustration above, the names are:
- tetrachlorcuprate(II)
- hexaaquairon(II)
- hexaamminecobalt(II)
- tetracyanonickelate(II)
- Notice in these examples that
- cuprate( Latin - cuprum) and nickelate are used in place of copper and nickel as they are anions
- Ammonia takes the prefix ammine as a ligand, which is spelt with a double 'm' unlike the functional group amine
Bidentate Ligands
- Bidentate ligands can each form two co-ordinate bonds to the central metal ion
- This is because each ligand contains two atoms with lone pairs of electrons
- Examples of bidentate ligands are:
- 1,2-diaminoethane (H2NCH2CH2NH2) which is also written as ‘en’
- Ethanedioate ion (C2O42- ) which is sometimes written as ‘ox’ (coming from the common name of oxalate)

Examples of complexes with bidentate ligands
Multidentate Ligands
- Some ligands contain more than two atoms with lone pairs of electrons
- These ligands can form more than two dative bonds and are said to be multidentate or polydentate ligands
- An example of a multidentate ligand is EDTA4-, which is a hexadentate ligand as it forms 6 dative covalent bonds to the central metal ion
- EDTA comes from ethylenediaminetetraacetic acid, which is rather a mouthful so EDTA is easier!
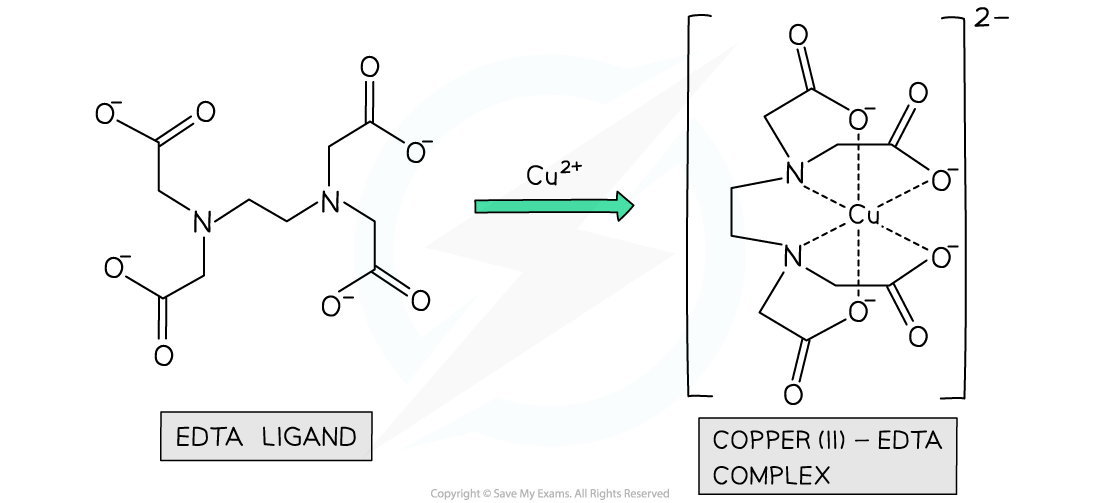
Example of a polydentate ligand complex
Deducing the Charge on a Complex Ion
- You can deduce the charge on a complex ion if you know the charges on the ligands and the oxidation state of the transition metal ion
Worked Example
The three formulas shown are compounds of chromium(III). What are the charges on the complex ions shown?
I [Cr(H2O)6]Cl3
II [CrCl(H2O)5]Cl2.H2O
III [CrCl2(H2O)4]Cl.2H2O
A 0 , 0 , 0
B 1+, 2+, 3+
C 2+, 3+, 1+
D 3+, 2+, 1+
Answer
The correct option is D
- The water molecules do not contribute to the charge
- The chloride ion, Cl-, outside the square brackets must balance against the charge on the complex
- [Cr(H2O)6]Cl3 contains three chloride ions, so the charge on the complex is 3+
- [CrCl(H2O)5]Cl2.H2O contains two chloride ions outside the square bracket, so the charge on the complex is 2+
- [CrCl2(H2O)4]Cl.2H2O contains one chloride ion outside the square bracket, so the charge on the complex is 1+
