Variable Oxidation States
- When transition elements forms ions they lose electrons from the 4s subshell first
- This is because when the orbitals are occupied, the repulsion between electrons pushes the 4s into a higher energy state so that it now becomes slightly higher in energy than the 3d subshell
- The 4s is now the outer shell and loses electrons first
- The loss of the 4s electrons means that +2 is a common oxidation state in transition metals
- The reason why the transition metals have variable oxidation states all comes down to energy
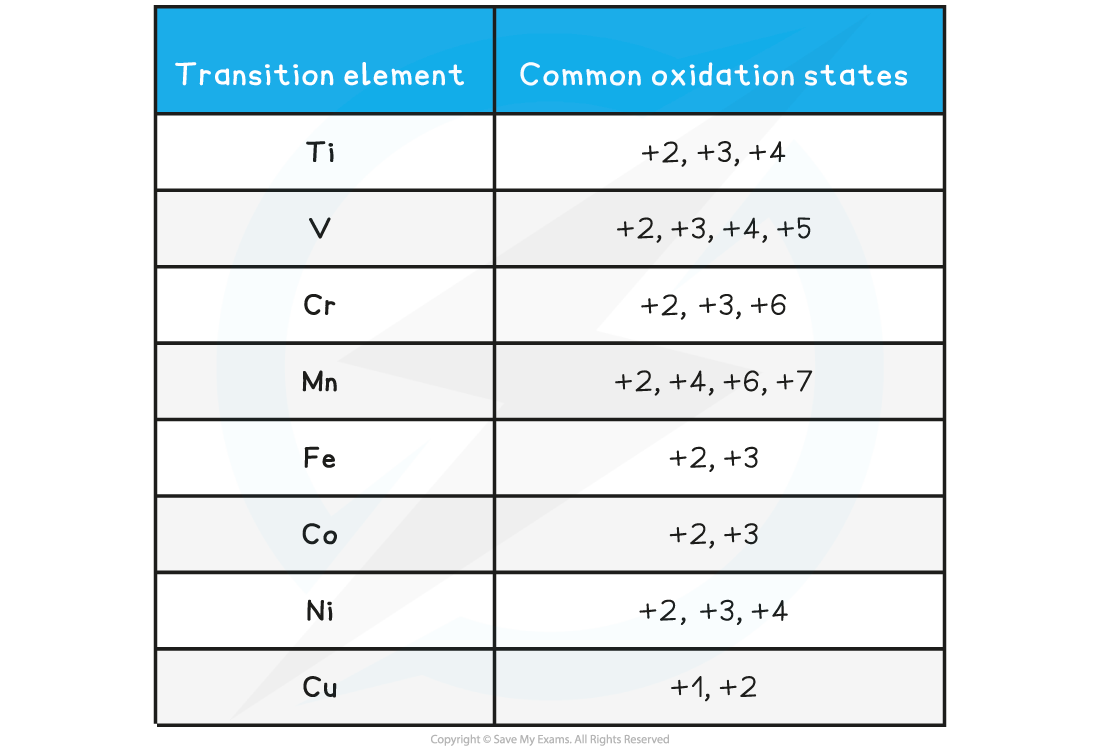
Table showing the the common oxidation states of transition elements
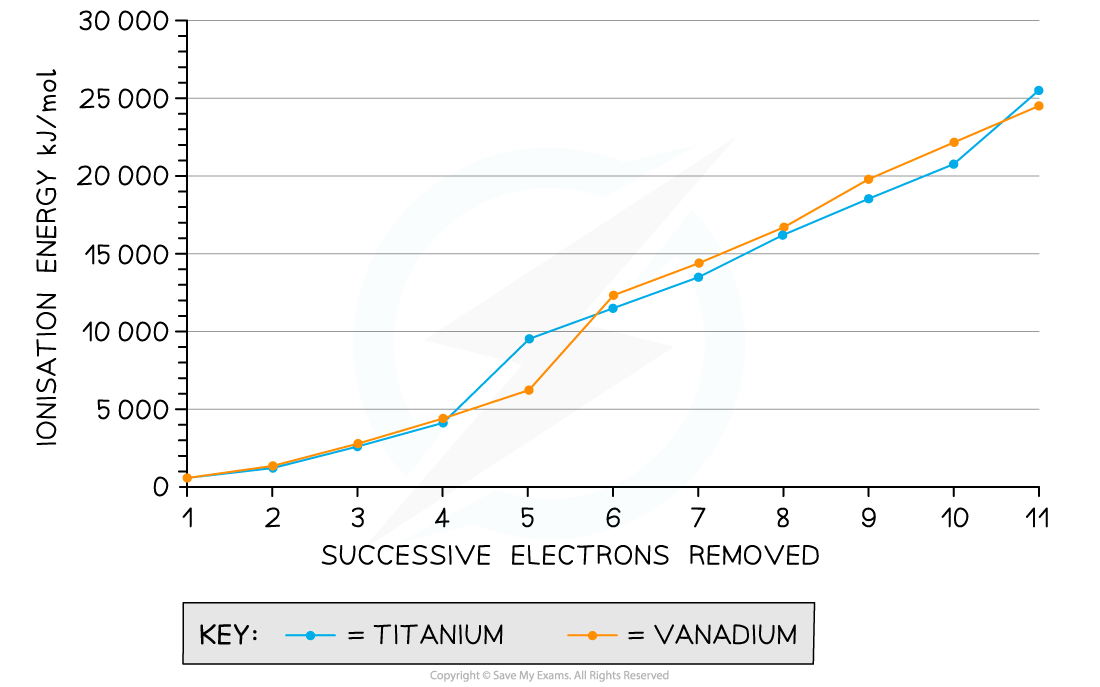
Ionisation energies for the removal of successive electrons in titanium and vanadium
- We can see from the graph that the first few ionisation energies are relatively small and relatively close together
- This means that the energy difference associated with removing a small number of electrons enables transition metals to vary their oxidation state with ease
- The +2 and +3 oxidation state is shown by all the transition elements although the +3 state is more stable up to chromium and the +2 state more stable in the later elements
- Transition metal ions with oxidation state +3 and above tend to be polarising and have a degree of covalent character in the bonds they form. The ions have a high charge density and pull electrons towards themselves
- The maximum oxidation state possible corresponds the total number of electrons in the 4s and 3d which reaches a maximum at manganese
- An example you may be familiar with is the manganate(VII) ion, MnO4- which is a powerful oxidising agent
Exam Tip
Common oxidation charges on transition metal ions are given in section 9 of the data booklet, and common oxidation states are given in section 14.
