Electroplating
- As we have seen, electrolysis with active electrodes involves the deposit of metals onto the surface of the cathode
- Electroplating involves the electrolytic coating of an object with a very thin metallic layer
- This is done for the purposes of decoration or for corrosion prevention
- For example, gold plated jewellery is made for aesthetic reasons and iron is galvanized with zinc to protect the iron from rusting
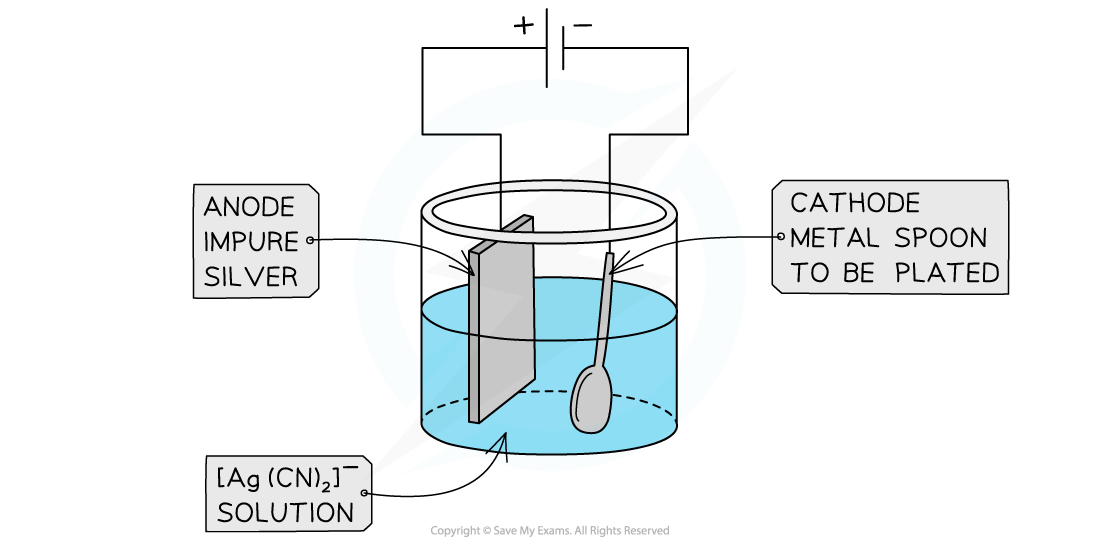
Electroplating an object with silver
- For successful electroplating, the metal needs to be deposited slowly and evenly
- The anode is usually made from the same metal to replenish the loss of the metal during electrolysis and maintain a constant concentration of the electrolyte
- Sodium silver cyanide, Na[Ag(CN)2], also know as sodium dicyanoargentate(I), is the preferred electrolyte for silver plating
- The reaction at the anode is:
Ag (s) + 2CN- (aq) → [Ag(CN)2]- (aq)+ e-
- The reaction at the cathode is:
[Ag(CN)2]- (aq) + e- → Ag (s) + 2CN- (aq)
- Impure silver is made the anode and it slowly dissolves away during the electrolysis
- By controlling the current, time and concentration of the electrolyte the rate of deposition can be carefully controlled to adjust the thickness of the metallic layer
- Even plastics have been electroplated using an ingenious process where the surface of the plastic is pitted and then imbedded with metallic particles that conduct electricity and can then be metal plated
