Ozone Revisited
- We have seen previously that ozone is a molecule with two resonance structures leading to a resonance hybrid

The two Lewis resonance structures for ozone
- The central oxygen atom has three electron domains and a lone pair, so the domain geometry is triangular planar and the molecular geometry is bent linear
- The presence of the lone pair repels the bonding pairs more strongly so the bond angle is reduced to 117o

The molecular structure of ozone
- The bond order for each bond in ozone is
bond order in O3 = total number of O3 bonding pairs ÷ total number of positions = 3 ÷ 2 = 1.5
- This gives a polar molecule with bonds that are weaker than the double bond in oxygen molecules

The structure of oxygen and ozone
- You would expect O-O bonds to be non-polar as the atoms have the same electronegativity; this is correct, but overall the molecule is polar due to the uneven distribution of electron cloud charge
- The formal charge on the Lewis structures show that the electrons are unevenly distributed
FC= (number of valence electrons) – ½(number of bonding electrons) – (number of non-bonding electrons)
FC (oxygen A) = (6) - ½(2) - (6) = -1
FC (oxygen B) = (6) - ½(6) - (2) = +1
FC (oxygen C) = (6) - ½(4) - (4) = 0

Formal charges on the oxygens in ozone
Catalytic Depletion
- The bonding and structure of ozone is key to understanding how the catalytic depletion of ozone occurs in the stratosphere
- High energy UV radiation in the stratosphere breaks the oxygen-oxygen double bond creating oxygen atoms
O2 (g) → O⋅ (g) + O⋅ (g) ∆H +ve, UV light, λ < 242 nm
- These oxygen atoms have unpaired electrons- they are known as free radicals
- The free radicals are highly reactive and quickly attack oxygen molecules forming ozone in an exothermic reaction, which raises the temperature of the stratosphere
OZONE FORMATION O⋅ (g) + O2 (g) → O3 (g) ∆H – ve
- Ozone requires less energy to break than oxygen
- It produces an oxygen molecule and an oxygen free radical:
OZONE DEPLETION O3 (g) → O⋅ (g) + O2 (g) ∆H +ve, UV light, λ< 330 nm
- The radical reacts with another ozone molecule making two molecules of oxygen in an exothermic reaction
OZONE DEPLETION O3 (g) + O⋅ (g) → 2O2 (g) ∆H – ve
- The temperature in the stratosphere is maintained by the balance of ozone formation and ozone depletion in a process known as the Chapman Cycle
- It is not a closed system as matter and energy flow in and out, but it is what is called a steady state
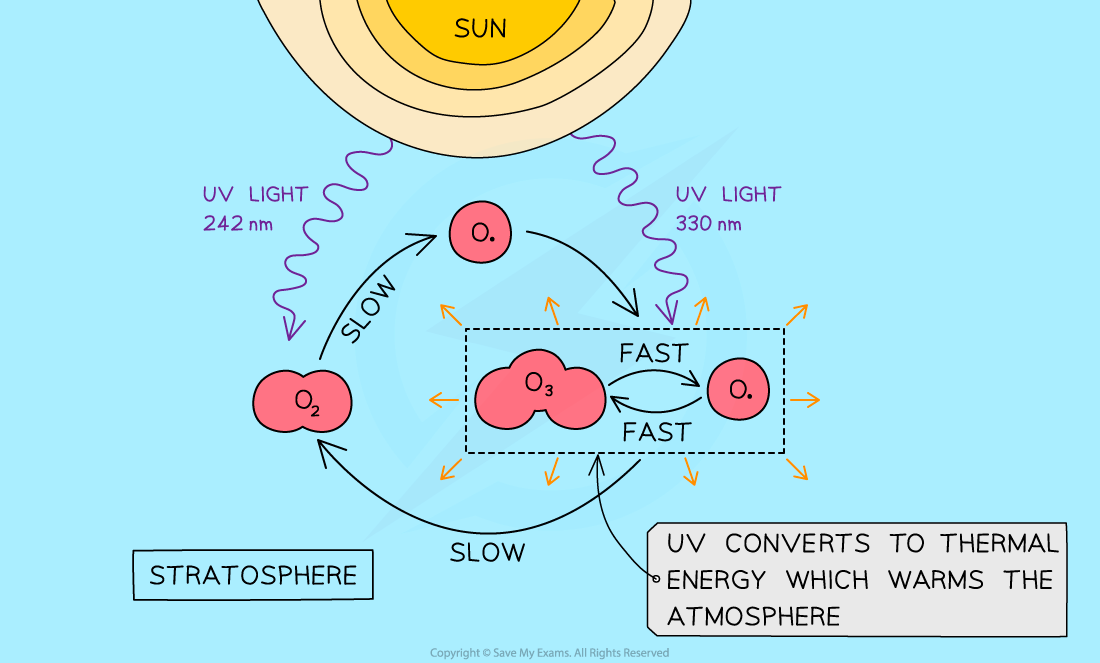
The Chapman cycle
Catalytic Depletion
- The two main man made culprits that accelerate the depletion of ozone are nitrogen oxides and CFCs
- Nitrogen monoxide, NO, is produced from the high temperatures inside internal combustion engines
- If you count the valence electrons in nitrogen monoxide (5 + 6 =11), the odd number tells you it is a free radical as it has an unpaired electron
- The nitrogen monoxide reacts with ozone forming oxygen and a nitrogen dioxide radical
NO⋅ (g) + O3 (g) → NO2⋅ (g) + O2 (g)
- The nitrogen dioxide produced is also a free radical (it has 5 + 6 + 6= 17 electrons)
NO2⋅ (g) + O⋅ (g) → NO⋅ (g) + O2 (g)
- The nitrogen monoxide is regenerated so it has a catalytic role in the process
- Combining the two equations and cancelling out the NO⋅ and NO2⋅ and you arrive at the overall depletion of ozone
O3 (g) + O⋅ (g) → 2O2 (g)
- A similar process happens with CFCs
- The C-Cl bond in the CFCs is weaker than the C-F bond and breaks more easily in the presence of UV light creating chlorine radicals
CCl2F2 (g) + UV → CClF2⋅ (g) + Cl⋅ (g)
- The chlorine radicals attack ozone and are regenerated at the end of the cycle
Cl⋅ (g) + O3 (g) → ClO⋅ (g) + O2 (g)
ClO⋅ (g) + O⋅ (g) → Cl⋅ (g) + O2 (g)
- Once again a molecule of ozone has been destroyed by a catalytic free radical
- The net effect of these reactions is that these pollutants have created an imbalance in the natural ozone cycle leading to an overall depletion in stratospheric ozone
- CFCs are greatly damaging to stratospheric ozone and have been largely replaced by safer alternatives following the 1985 Montreal Protocol
- The depletion of ozone has allowed greater amounts of harmful UV light to reach the surface of the Earth
- UV light has been linked to greater incidence of skin cancer and cataracts as well as the destruction of phytoplankton and reduced plant growth
