Rate of Reaction
Reaction rate
- Some reactions take place instantly, but most are much slower and it is possible to measure how long these reactions take to reach a certain stage
- As a chemical reaction proceeds, the concentration of the reactants decreases and the concentration of the products increases
- The rate of a reaction is the speed at which a chemical reaction takes place and has units mol dm-3 s-1
- The rate of a reaction can be calculated by:

- Graphically we can represent the rate of reaction as:

Rate of reaction graphs
Worked Example
Iodine and methanoic acid react in aqueous solution.
I2 (aq) + HCOOH (aq) → 2I− (aq) + 2H+ (aq) + CO2 (g)
The rate of reaction can be found by measuring the volume of carbon dioxide produced per unit time and plotting a graph as shown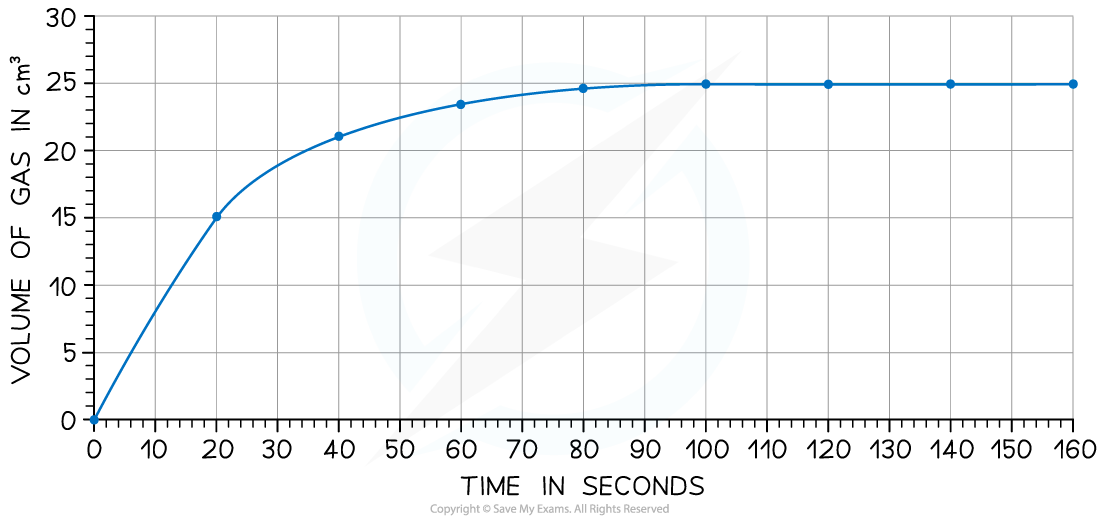 Calculate the rate of reaction at 20 seconds
Calculate the rate of reaction at 20 secondsAnswer:
- Draw a tangent to the curve at 20 seconds:
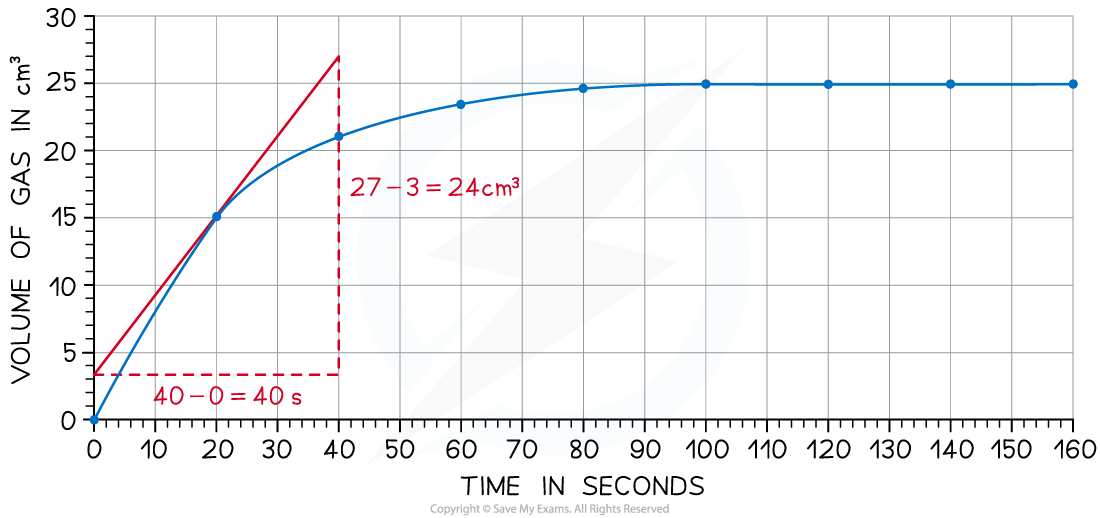
- Complete the triangle and read off the values of x and y
- Determine the gradient of the line using ∆y / ∆x
- Rate of reaction = 24 ÷ 40 = 0.60 cm3 s-1
Exam Tip
When drawing the tangent to a curve make the triangle large and try to intersect with gridlines if you can. This minimises errors of precision and reduces the chance you will accidently misread the graph values
