Temperature & Kinetic Energy
- What is the difference between heat and temperature?
- This can be illustrated using a beaker of boiling water and a pipette:
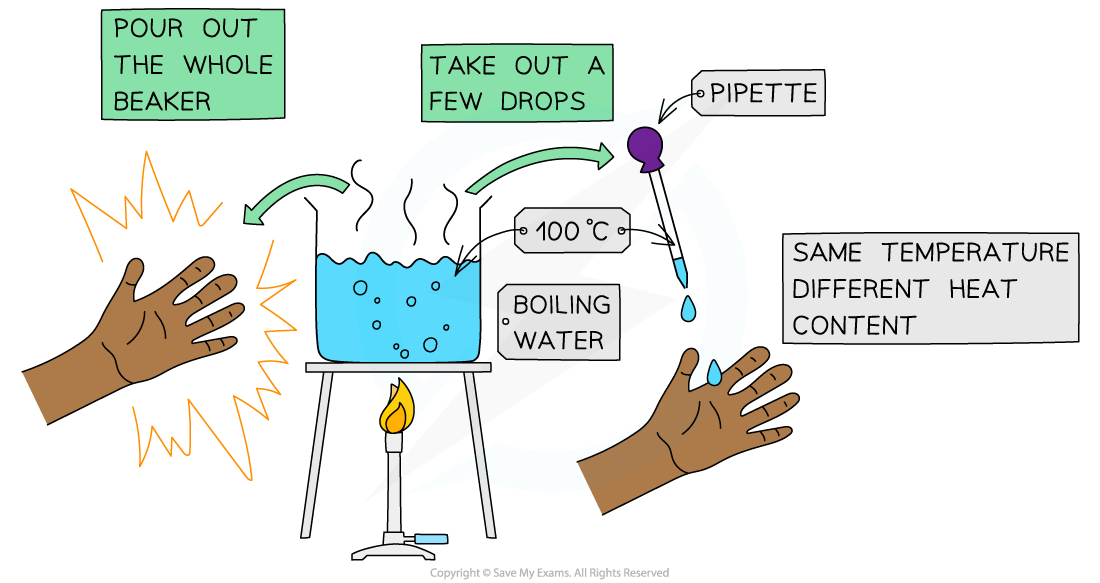
The effects of boiling water can be quite different depending on the quantity of water involved!
- You would get a very nasty burn if a whole beaker of boiling water spilled onto to your hand, but a drop of boiling water would cause little problem
- The water is at the same temperature in the pipette as in the beaker, but the beaker has a much higher heat content
- We say that temperature is a measure of the average kinetic energy of the particles whereas heat is a measure of the energy content of a substance
- The particles have kinetic energy because they are moving
- The faster they move the more energy they have and the higher the temperature of the substance
Conservation of Energy
- Energy is a measure of the ability to do work
- There are many different types of energy and heat is only one of them
- During chemical reactions energy flows in and out of the reaction vessels
- Inside the reaction vessel is known as the system
- Outside the reaction vessel is known as the surroundings
- Systems come in three types: open, closed and isolated:
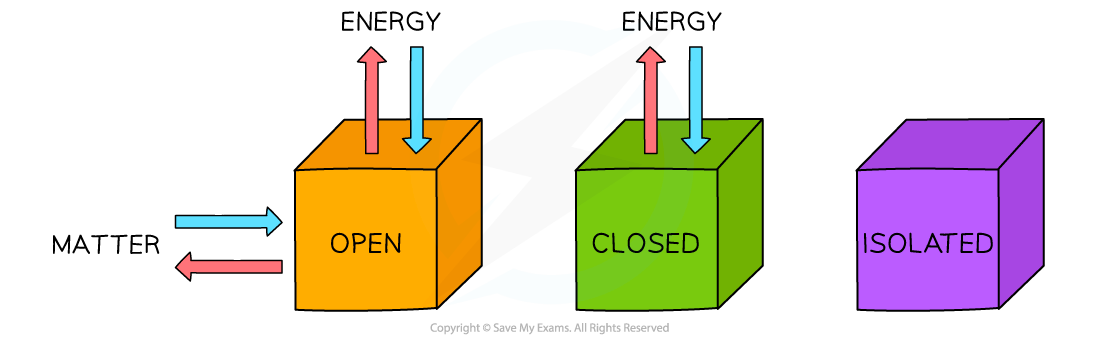
Three types of systems
- Isolated systems are very rare; most chemical reactions are open systems
- Open systems are very important when thinking about chemical equilibrium which is covered in Topic 7
- Although energy can be exchanged between open and closed systems and the surroundings, the total energy of the process cannot change
- This is known as the Law of Conservation of Energy and is a cornerstone to understanding how chemical changes affect the energy flow in and out of systems
Exothermic & Endothermic
- The total chemical energy inside a substance is called the enthalpy (or heat content)
- When chemical reactions take place, changes in chemical energy take place and therefore the enthalpy changes
- An enthalpy change is represented by the symbol ΔH (Δ= change; H = enthalpy)
- An enthalpy change can be positive or negative
Exothermic reactions
- A reaction is exothermic when the products have less enthalpy than the reactants
- Heat energy is given off by the system to the surroundings
- The temperature of the surroundings increases
- The temperature of the system decreases
- There is an enthalpy decrease during the reaction so ΔH is negative
- Exothermic reactions are thermodynamically possible (because the enthalpy of the reactants is higher than that of the products)
- However, if the rate is too slow, the reaction may not occur. In this case the reaction is kinetically controlled
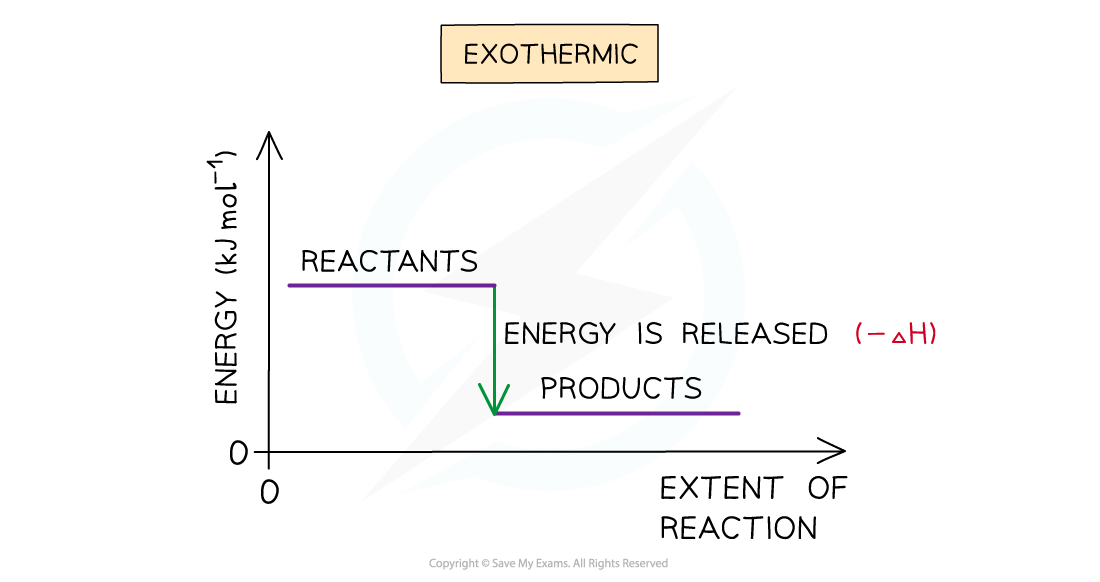
The enthalpy change during an exothermic reaction
Endothermic reactions
- A reaction is endothermic when the products have more enthalpy than the reactants
- Heat energy is absorbed by the system from the surroundings
- The temperature of the surroundings decreases
- The temperature of the system increases
- There is an enthalpy increase during the reaction so ΔH is positive
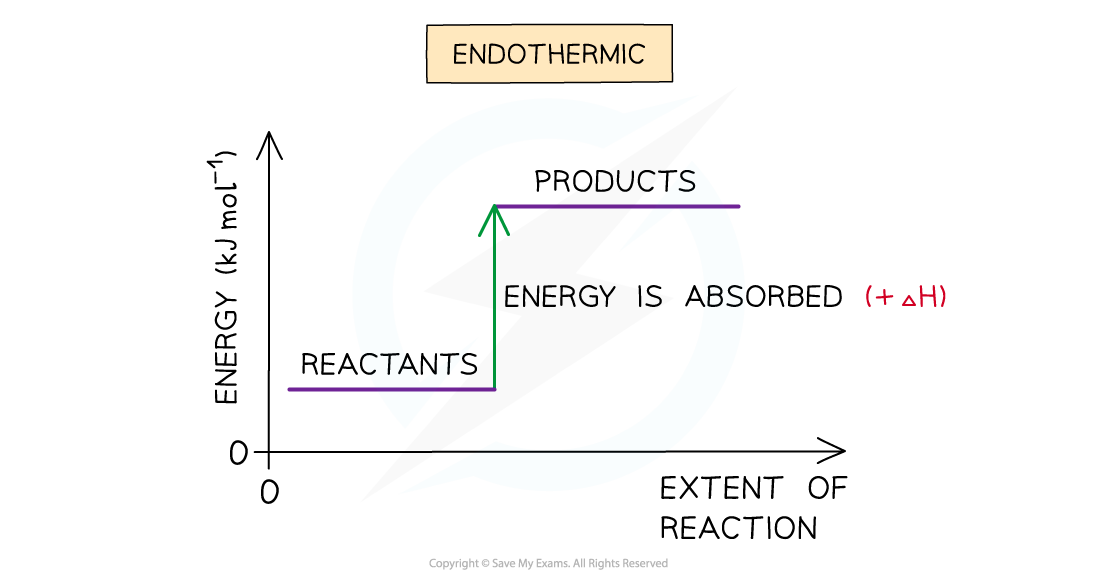
The enthalpy change during an endothermic reaction
Exam Tip
It is important to specify the physical states of each species in an equation when dealing with enthalpy changes as any changes in state can cause very large changes of enthalpy.For example:
NaCl (s) → Na+ (aq) + Cl- (aq) ΔH = +4 kJ mol-1
NaCl (s) → Na+ (g) + Cl- (g) ΔH = +787 kJ mol-1
Also, remember that the system is the substances that are reacting (ie. the reaction itself) and the surroundings is everything else (eg. the flask the reaction is taking place in)
