Covalent Bonds
- Covalent bonding occurs between two non-metals
- A covalent bond involves the electrostatic attraction between nuclei of two atoms and the electrons of their outer shells
- No electrons are transferred but only shared in this type of bonding
- When a covalent bond is formed, two atomic orbitals overlap and a molecular orbital is formed
- Covalent bonding happens because the electrons are more stable when attracted to two nuclei than when attracted to only one
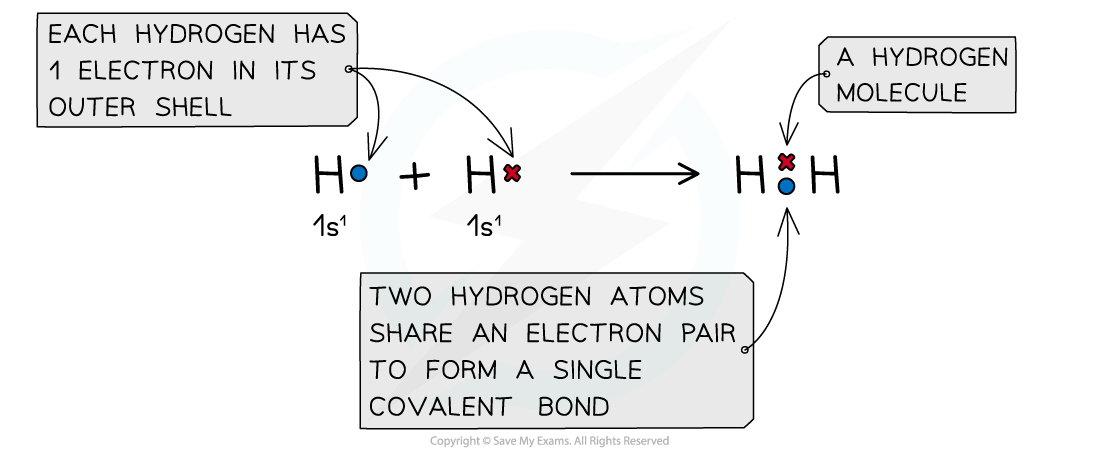
The positive nucleus of each atom has an attraction for the bonding electrons shared in the covalent bond
- In a normal covalent bond, each atom provide one of the electrons in the bond. A covalent bond is represented by a short straight line between the two atoms, H-H
- Covalent bonds should not be regarded as shared electron pairs in a fixed position; the electrons are in a state of constant motion and are best regarded as charge clouds
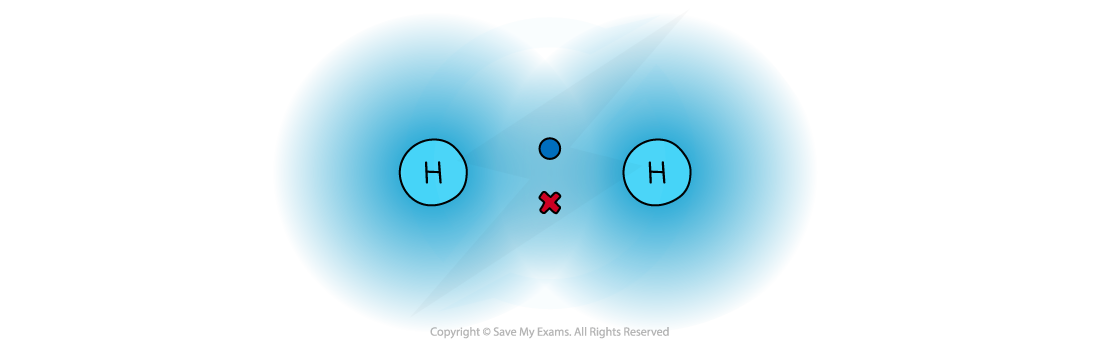
A representation of electron charge clouds. The electrons can be found anywhere in the charge clouds
- Non-metals are able to share pairs of electrons to form different types of covalent bonds
- Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
- This makes each atom more stable
- In some instances, the central atom of a covalently bonded molecule can accommodate more or less than 8 electrons in its outer shell
- Being able to accommodate more than 8 electrons in the outer shell is known as ‘expanding the octet rule’
- Accommodating less than 8 electrons in the outer shell means than the central atom is ‘electron deficient’
- Some examples of this can be found in the section on Lewis structures
Exam Tip
Covalent bonding takes place between two nonmetal atoms.Remember to use the periodic table to decide how many electrons are in the outer shell of a nonmetal atom.
Predicting Covalent Bonding
- The differences in Pauling electronegativity values can be used to predict whether a bond is covalent or ionic in character
Electronegativity & covalent bonds
- In diatomic molecules the electron density is shared equally between the two atoms
- Eg. H2, O2 and Cl2
- Both atoms will have the same electronegativity value and have an equal attraction for the bonding pair of electrons leading to formation of a covalent bond
- A difference of less than around 1.0 in electronegativity values will be associated with covalent bonds, although between 1.0 and 2.0 can be considered polar covalent:
You can use the Pauling scale to decide whether a bond is polar or nonpolar: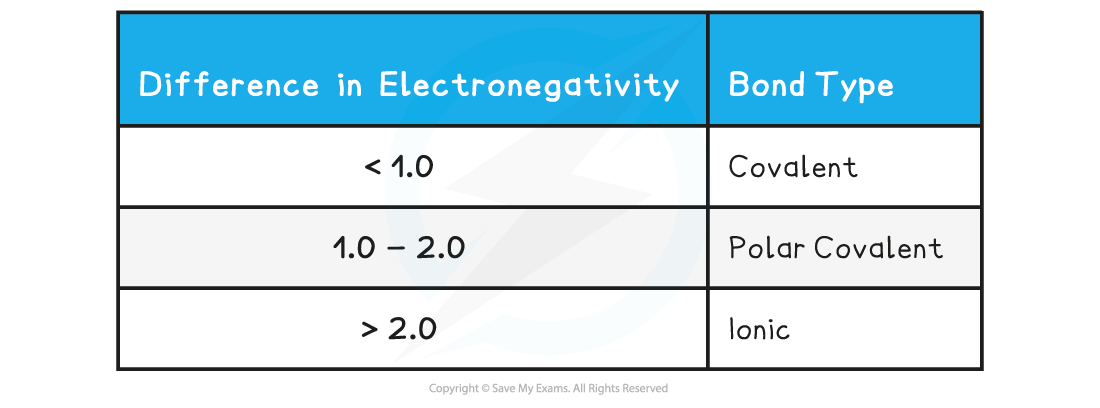
Coordinate Bonds
- In simple covalent bonds the two atoms involved share electrons
- Some molecules have a lone pair of electrons that can be donated to form a bond with an electron-deficient atom
- An electron-deficient atom is an atom that has an unfilled outer orbital
- So both electrons are from the same atom
- This type of bonding is called dative covalent bonding or coordinate bond
- An example of a dative bond is in an ammonium ion
- The hydrogen ion, H+ is electron-deficient and has space for two electrons in its shell
- The nitrogen atom in ammonia has a lone pair of electrons which it can donate to the hydrogen ion to form a dative covalent bond
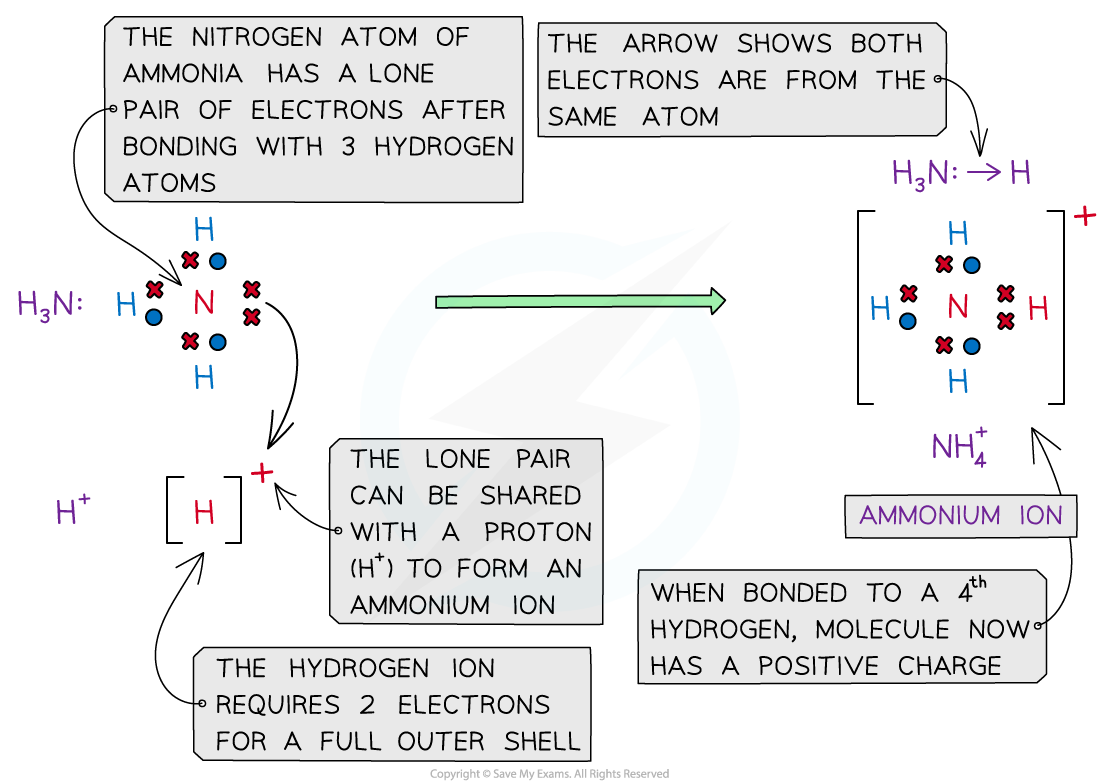
Ammonia (NH3) can donate a lone pair to an electron-deficient proton (H+) to form a charged ammonium ion (NH4+)
- More examples of coordinate bonding can be found in the section on Lewis Structures
Multiple Bonds
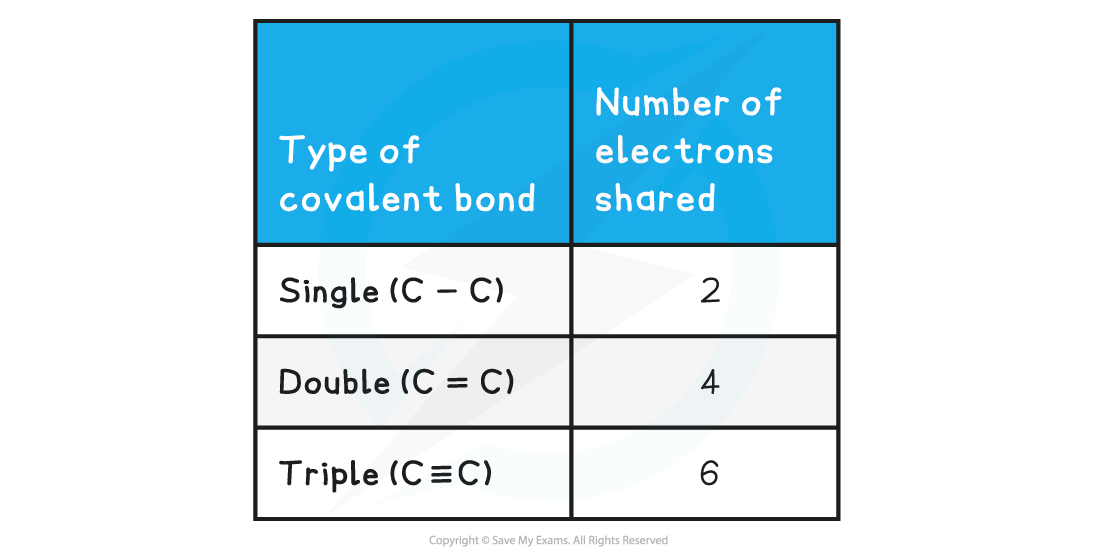
- Non-metals are able to share more than one pair of electrons to form different types of covalent bonds
- Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
- This makes each atom more stable
- It is not possible to form a quadruple bond as the repulsion from having 8 electrons in the same region between the two nuclei is too great
Covalent Bonds & Shared Electrons Table
Bond Length & Strength
Bond energy
- The bond energy is the energy required to break one mole of a particular covalent bond in the gaseous states
- Bond energy has units of kJ mol-1
- The larger the bond energy, the stronger the covalent bond is
Bond length
- The bond length is internuclear distance of two covalently bonded atoms
- It is the distance from the nucleus of one atom to another atom which forms the covalent bond
- The greater the forces of attraction between electrons and nuclei, the more the atoms are pulled closer to each other
- This decreases the bond length of a molecule and increases the strength of the covalent bond
- Triple bonds are the shortest and strongest covalent bonds due to the large electron density between the nuclei of the two atoms
- This increase the forces of attraction between the electrons and nuclei of the atoms
- As a result of this, the atoms are pulled closer together causing a shorter bond length
- The increased forces of attraction also means that the covalent bond is stronger

Triple bonds are the shortest covalent bonds and therefore the strongest ones
- Test your knowledge of covalent bonding:
Worked Example
Which molecules react together to form a dative covalent bond?
A. Cl2 and HF
B. C2H2 and Cl2
C. NH3 and HF
D. CH4 and NH3
Answer:
The correct option is C.
- To form a dative covalent bond one species must have a lone pair of electrons and the other must be electron deficient.
- NH3 has a lone pair and HF splits into H+ (electron deficient) and F-
NH3 + HF → NH4+ F-
