Nomenclature
- Systematic nomenclature can be used to name organic compounds and therefore make it easier to refer to them
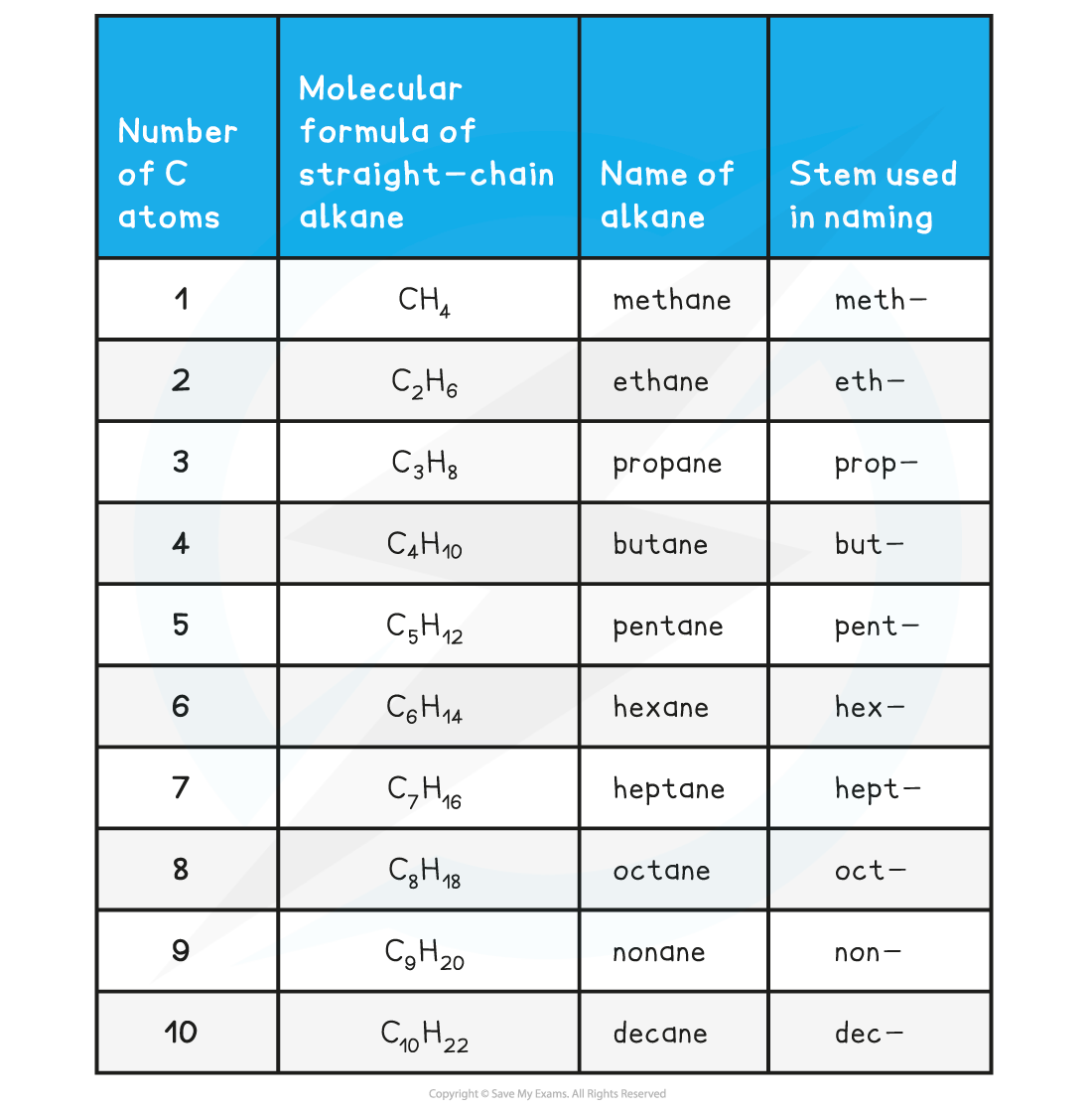
- The alkanes provide the basis of the naming system and the stem of each name indicates how many carbon atoms are in the longest chain in one molecule of the compound
Nomenclature of Organic Compounds Table
Exam Tip
Although the table shows up to 10 carbons for reference, in your IB Chemistry exam you are only required to name molecules with up to 6 carbons
Chains & Branches
- If there are any side-chains or functional groups present, then the position of these groups are indicated by numbering the carbon atoms in the longest chain starting at the end that gives the lowest possible numbers in the name
- The hydrocarbon side-chain is shown in brackets in the structural formula
CH3CH(CH3)CH2CH3
- The side-chain is named by adding ‘-yl’ to the normal alkane stem
- This type of group is called an alkyl group

Naming Side Chains
- If there are more than one of the same alkyl side-chain or functional groups, di- (for two), tri- (for three) or tetra- (for four) is added in front of its name
- The adjacent numbers have a comma between them
- Numbers are separated from words by a hyphen

Naming Multiple Side Chains
- If there is more than one type of alkyl side-chain, they are listed in alphabetic order

Naming Side Chains in Alphabetical Order
Exam Tip
An aliphatic compound is straight or branched-chain and also includes cyclic organic compounds that do not contain a benzene ring
