Resonance Structures
- The delocalization of electrons can explain the structures of some species that don’t seem to fit with a Lewis structure
- Delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond
- The Lewis diagram for the nitrate (V) ion gives a molecule with a double and two single bonds
- There are three possible Lewis Structures
- These structures are called resonance structures
- However, studies of the electron density and bond length in the nitrate (V) ion indicate all the bonds are equal in length and the electron density is spread evenly between the three oxygen atoms
- The bond length is intermediate between a single and a double bond
- The actual structure is something in between the resonance structures and is known as a resonance hybrid
Resonance structures of the nitrate (V) ion
- To determine the Lewis structure of the nitrate (V) ion first count the number of valence electrons and then add one electron for the negative charge on the ion
Number of valence electrons = N + 3O +1
= 5 + ( 3 x 6) + 1 = 24 electrons
- Three structures are possible, consisting of a double bond and two singles:
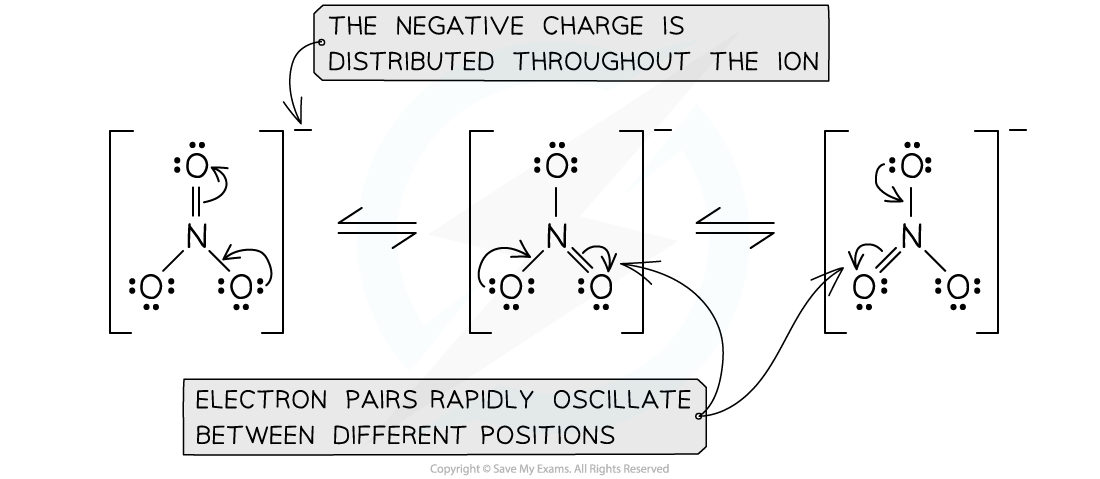
Resonance structures in the nitrate ion
- Dotted lines are used to show the position of the delocalised electrons

Resonance hybrid nitrate (V) ion
- The criteria for forming resonance hybrids structures is that molecules must have a double bond (pi bond) that is capable of migrating from one part of a molecule to another
- This usually arises when there are adjacent atoms with equal electronegativity and lone pairs of electrons that can re-arrange themselves and allow the double bonds to be in different positions
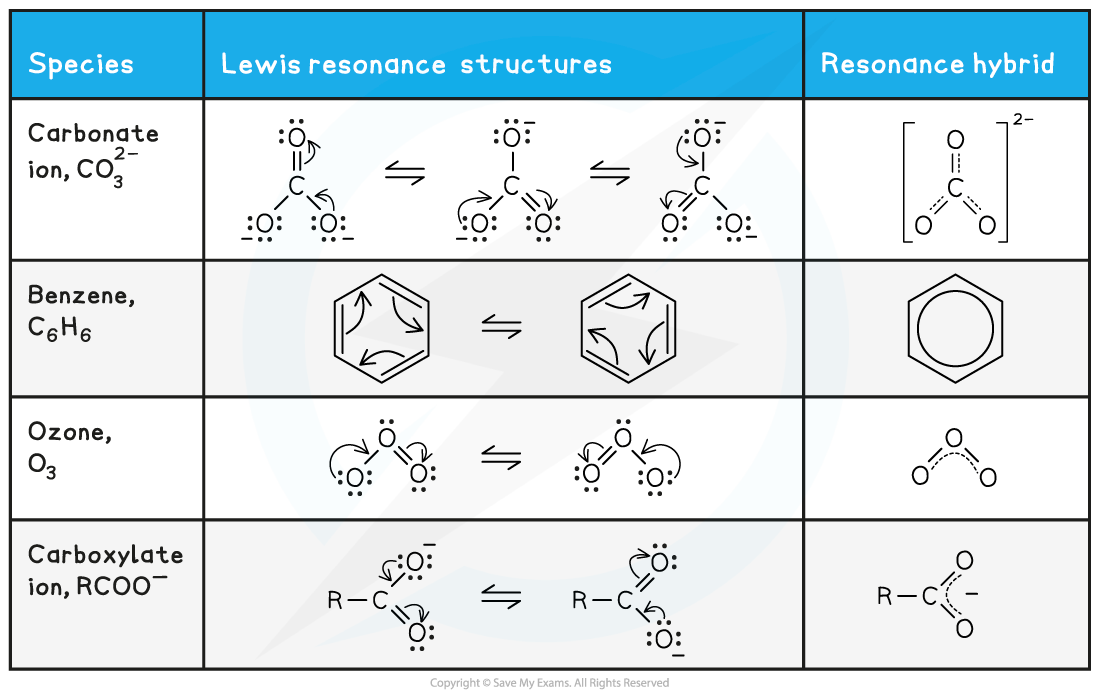
- Other examples that you should know about are the carbonate ion, benzene, ozone and the carboxylate anion
Resonance Hybrids Table
- Below are some other resonance structures and hybrids that you should know: