Further VSEPR Theory
Revisiting Valence Shell Electron Pair Repulsion Theory (VSEPR)
- When an atom forms a covalent bond with another atom, the electrons in the different bonds and the non-bonding electrons in the outer shell all behave as negatively charged clouds and repel each other
- In order to minimise this repulsion, all the outer shell electrons spread out as far apart in space as possible
- Molecular shapes and the angles between bonds can be predicted by the valence shell electron pair repulsion theory known by the abbreviation VSEPR theory
- VSEPR theory consists of three basic rules:
- All electron pairs and all lone pairs arrange themselves as far apart in space as is possible.
- Lone pairs repel more strongly than bonding pairs
- Multiple bonds behave like single bonds
- These three rules can be used to predict the shape of any covalent molecule or ion, and the angles between the bonds
- The regions of negative cloud charge are known as domains and can have one, two or three pairs electrons
Molecular geometry versus domain geometry
- It is important to distinguish between molecular geometry and domain geometry in exam questions
- Molecular geometry refers to the shape of the molecules based on the relative orientation of the atoms
- Domain geometry refers to the relative orientation of all the bonding and lone pairs of electrons
- The Lewis structure for water enables us to see that there are four electron pairs around the oxygen so the domain geometry is tetrahedral
- However, the molecular geometry shows us there are two angled bonds so the shape is bent, angular, bent linear or V-shaped (when viewed upside down)
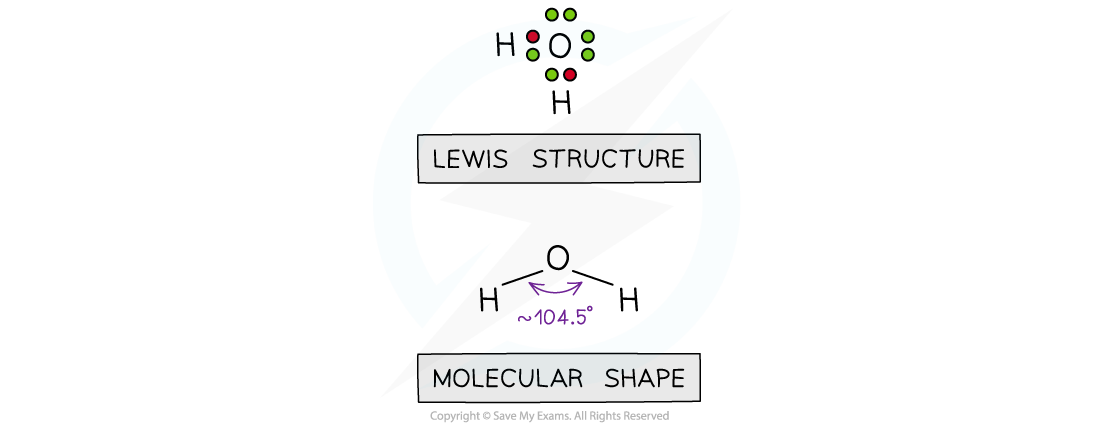
Diagram showing the Lewis structure of water and molecular shape from which the domain and molecular geometries may be determined
Five electron domains
Table showing the four molecular geometries associated with five electron domains
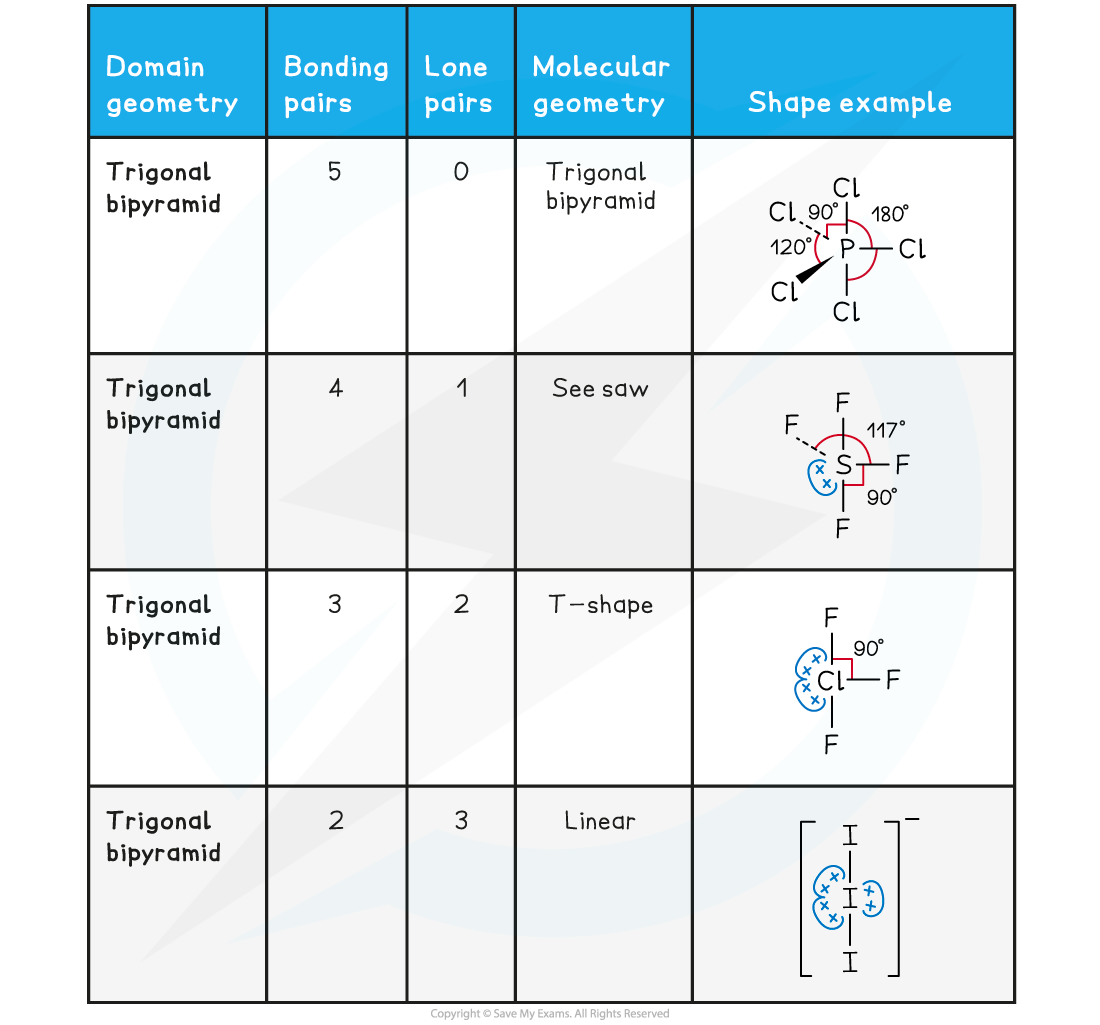
*Trigonal or triangular may be used
- Notice that PCl5 is a symmetrical molecule so the electron cloud charge is evenly spread
- This means that it will be a non-polar molecule as any dipoles from the P-Cl bonds would be cancelled out
- SF4, ClF3 are asymmetrical molecules having one or two lone pairs on one side of the central axis making the overall molecule polar
Six electron domains
Table showing the three molecular geometries associated with six electron domains
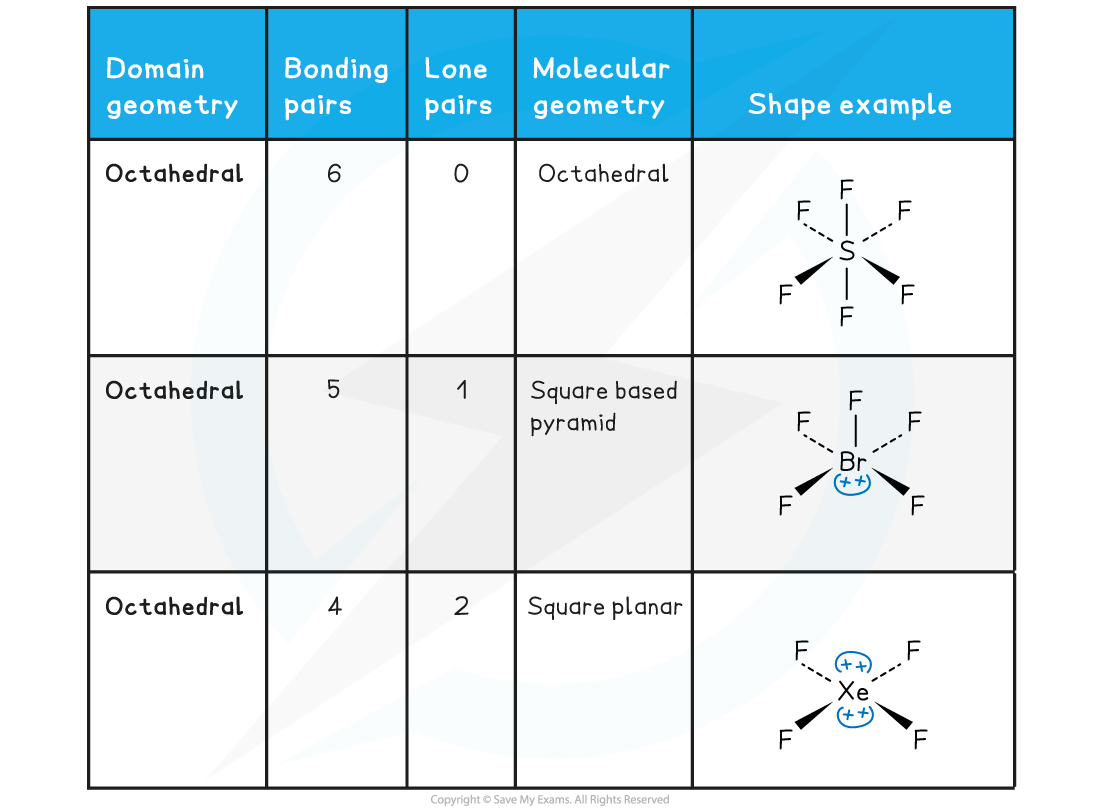
- SF6 is a symmetrical molecule so the electron cloud charge is evenly spread with 90o between the bonds
- This means that it will be a non-polar molecule as any dipoles from the S-F bonds would be cancelled out
- XeF4 is also non-polar despite having two lone pairs.
- The bonding pairs are at 90o to the plane and the lone pairs are at 180o
- The lone pairs are arranged above and below the square plane resulting in an even distribution of electron cloud charge
- BrF5 is asymmetrical having a lone pair at the base of the pyramid making the overall molecule polar
Worked Example
What is the domain geometry, molecular geometry and F-Xe-F bond angle of xenon difluoride, XeF2?
Answer
- Count the valence electrons = Xe + 2F = 8 + (2 x 7) = 22
- There are two bonding pairs, accounting for 4 electrons, so 18 electrons remain
- Each fluorine should have 3 lone pairs, accounting for 6 pairs or 12 electrons, which leaves 3 lone pairs on the xenon
- Xenon therefore has 2 bonding pairs and 3 lone pairs making its domain geometry trigonal pyramid and its molecular geometry linear
- The bond angle will be 180o (having the same structure as the triiodide ion)
