Conformational & Configurational Isomers
- Isomers are compounds that have the same molecular formula but a different arrangement of atoms
- Isomers can be grouped into various categories, as shown:

Flow chart of the various isomers with points to help identify them
- At Standard Level, we encountered three types of structural isomers:
- Functional group isomers, e.g. propanal and propanone
- Position isomers, e.g. propan-1-ol and propan-2-ol
- Branch-chain isomers, e.g. butane and methylpropane
- If the atoms within an isomer are arranged in the same order then we are dealing with stereoisomers
- Stereoisomers can be conformational or configurational
Conformational Isomers
- Conformational isomers, or conformers, occur due to free rotation about a single σ-bond and can be described as:
- Staggered
- Eclipsed
- One of the simplest examples of conformational isomerism is ethane, CH3CH3

Three-dimensional structure of ethane identifying the bond for conformational isomerism
- By looking along the C-C bond highlighted in the diagram we can draw the two Newman projections, staggered and eclipsed
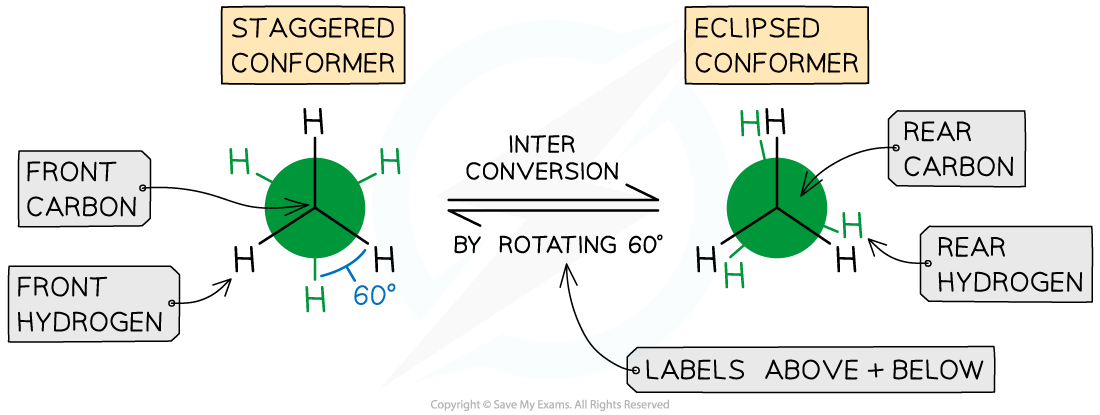
The staggered and eclipsed conformers of ethane
- The staggered conformer has angles between hydrogen atoms on adjacent carbons of 60o, as shown
- It is also more stable / lower energy than the eclipsed conformer because the C-H bonds are as far apart as possible to minimise the repulsion between the electrons in the C-H bonds
- The eclipsed conformer has angles between hydrogen atoms on adjacent carbons of 0o, this is not shown in the diagrams so that the conformation can be seen
- The eclipsed conformer is less stable / higher energy due to the repulsion between the electrons in the C-H bonds that are closer together
- The free rotation that causes these conformers means that it is easy to interconvert from one conformer to the other and back
- This is also the reason that it is almost impossible to isolate a single conformer
Conformational Isomerism in Cyclic Structures
- Conformational isomerism can also be seen in cyclic structures
- A common example of this is cyclohexane, C6H12
- Cyclohexane isomers exist in boat and chair forms:

Skeletal structures showing the boat and chair forms of cyclohexane
- The boat form is less stable / higher energy as there are four eclipsed bonds causing strain on the overall structure
- There is also repulsion of the hydrogen atoms on the end of the boat structure
- It is possible to "flip" between the boat and chair forms which explains the difficulty in isolating just one of the forms
- During the interconversions, it also possible to get other structures commonly called the half chair and the twisted boat
Configurational Isomers
- Interconversion of configurational isomers can only occur by breaking bonds or rearranging stereocentres
- Configurational isomers can be divided into:
- cis / trans isomers and E / Z isomers
- optical isomers
