Rate Constants
- The rate constant, (k), of a reaction can be calculated using the initial rates and the rate equation
Calculating the rate constant from the initial rate
- The reaction of sodium carbonate with chloride ions (from hydrochloric acid) to form sodium chloride will be used as an example to calculate the rate constant from the initial rate and initial concentrations
- The reaction and rate equation are as follows:
Na2CO3 (s) + 2Cl- (aq) + 2H+ (aq) → 2NaCl (aq) + CO2 (g) + H2O (l)

- The progress of the reaction can be followed by measuring the initial rates of the reaction using various initial concentrations of each reactant
Experimental results of concentrations & initial rates table
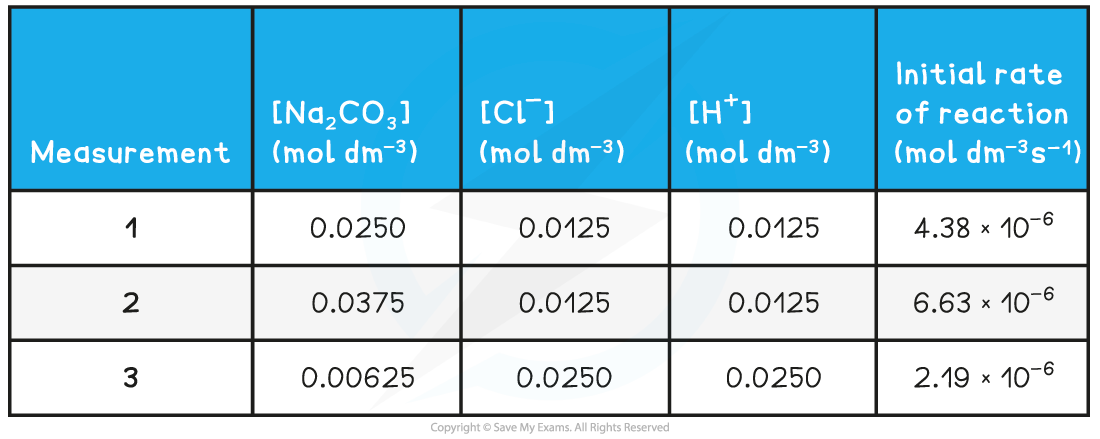
- To find the rate constant (k):
- Substitute the values of one of the experiments to find k (for example measurement 1)

- The values of measurement 2 or 3 could also have been used to find k
- They all give the same result of 1.40 x 10-2
Calculating units
- When you are asked to calculate the rate constant, k, for a reaction you must also be able to deduce the units
- This is done by replacing the values in the rearranged rate equation with the units of that value
- The units can then be combined or cancelled as required
- For example, to calculate the units for the above reaction:

Temperature and the rate constant, k
- The following general reaction and rate equation will be used to discuss the effect of temperature on the rate constant, k:
A + B → C + D
Rate of reaction = k[A][B]
- The rate equation shows that rate of reaction depends on the rate constant, k, and the concentration of the reactants
- As the rate of reaction increases the rate constant will increase
- Increasing the temperature of a reaction increases the rate of a chemical reaction
- Remember: this does not necessarily increase the yield of a chemical reaction depending on whether a reaction is endothermic or exothermic according to Le Châtelier’s principle
- Therefore, increasing the temperature also increases the value of the rate constant, k, assuming that the concentration of the reactants remains unchanged
- An exponential relationship between the rate of reaction and temperature is observed when seen on a graph:

Relationship between temperature and rate constant, k
- The graph shows that the rate of reaction roughly doubles with an increase of 10 oC
- This general relationship does not apply to all reactions
- Also, it is not necessarily every 10 oC, the rate may double every 9 °C or 11 °C
- The number of degrees needed to double the rate also changes gradually as temperature increases
