Graphing the Arrhenius Equation
Finding the Activation Energy
- Very often, the Arrhenius equation is used to calculate the activation energy of a reaction
- A question will either give sufficient information for the Arrhenius equation to be used or a graph can be plotted and the calculation done from the plot
Using the equation:
- Remember, it is usually easier to use the version of the Arrhenius equation after natural logs of each side have been taken
Using an Arrhenius plot:
- A graph of ln k against 1/T can be plotted, and then used to calculate Ea
- This gives a line which follows the form y = mx + c
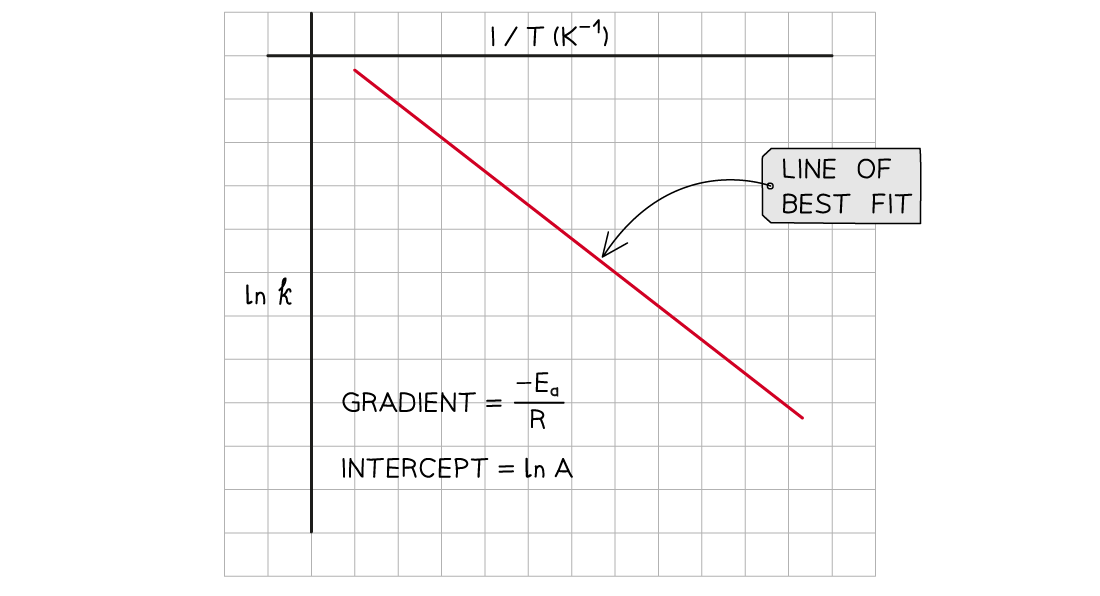
The graph of ln k against 1/T is a straight line with gradient -Ea/R
- From the graph, the equation in the form of y = mx + c is as follows:
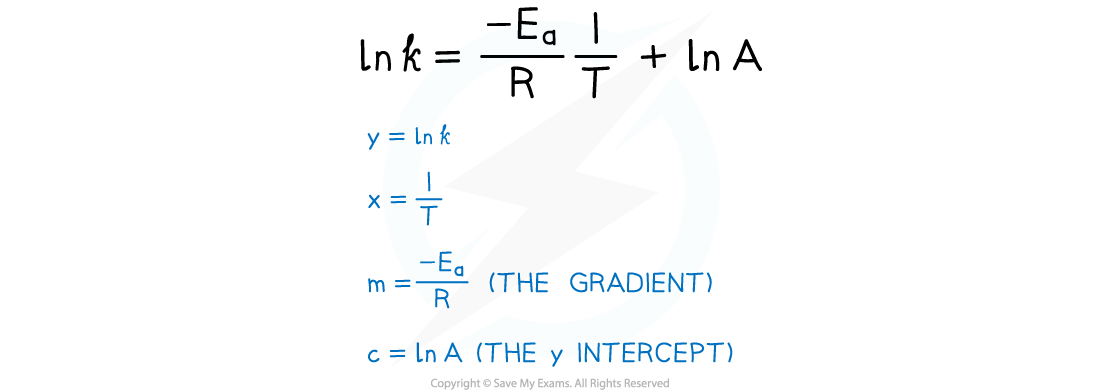
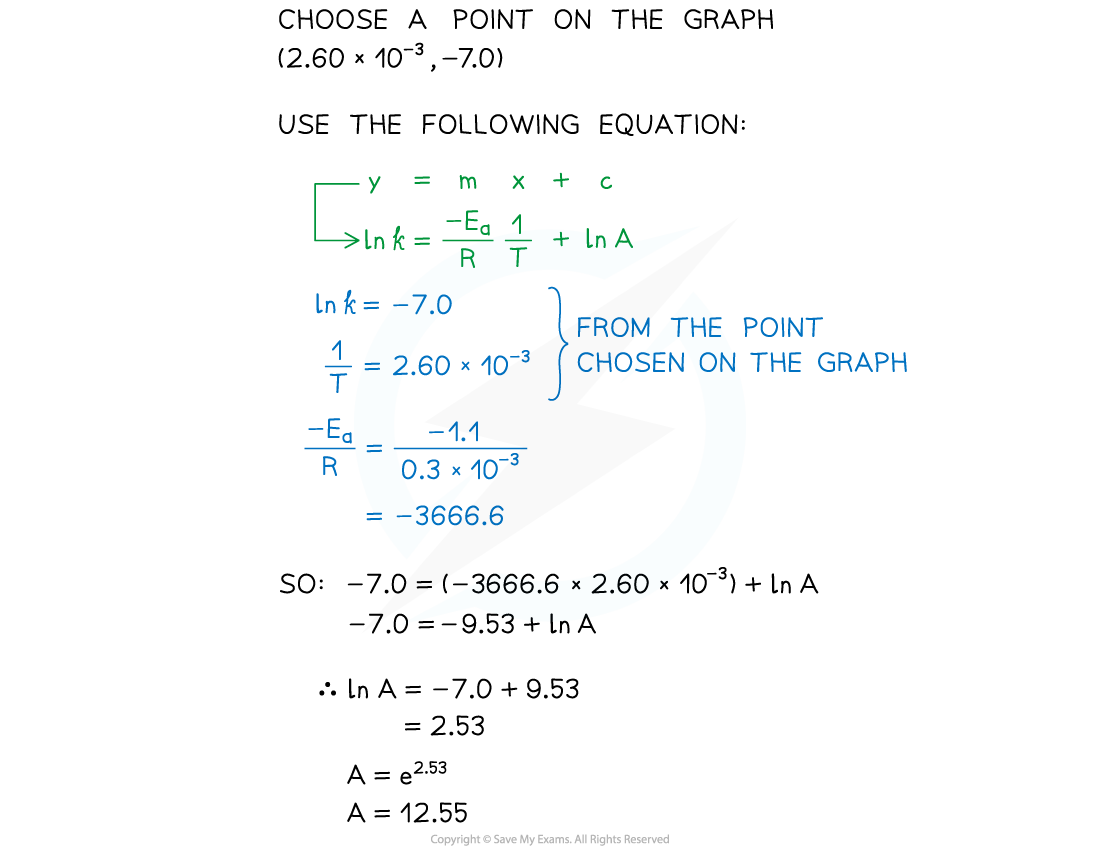
Worked Example
- Complete the following table
- Plot a graph of ln k against 1/T
- Use this to calculate the activation energy, Ea, and the Arrhenius constant, A, of the reaction.
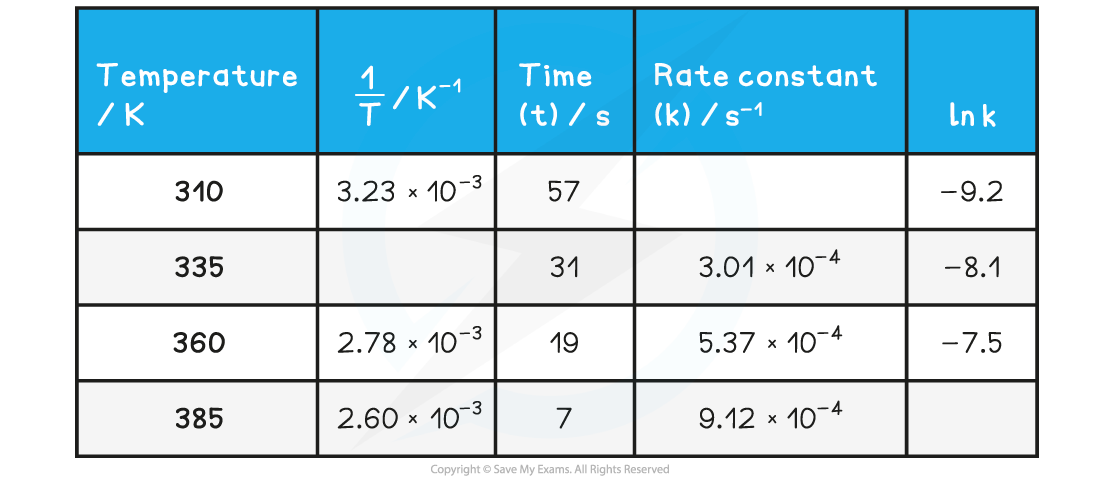
Answers
Answer 1:
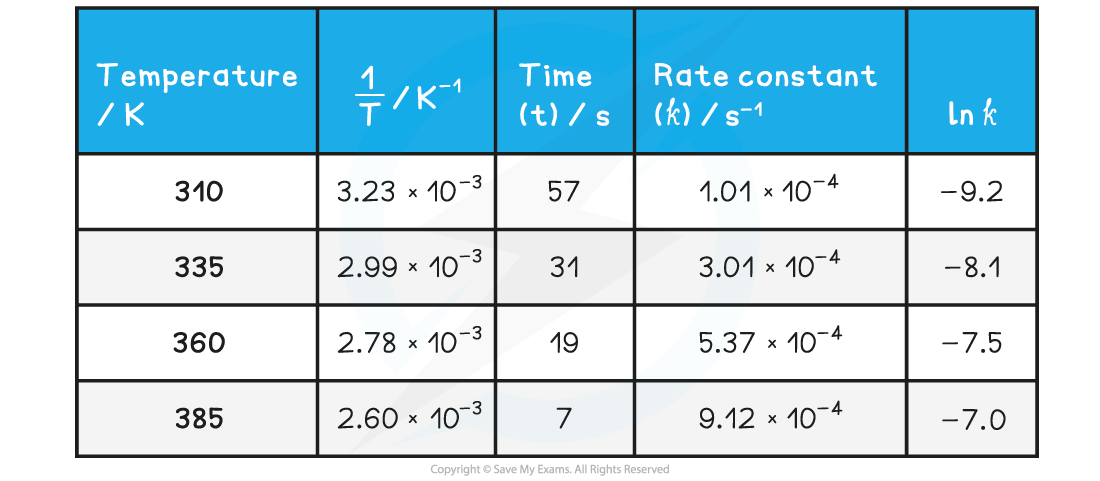
Answer 2:
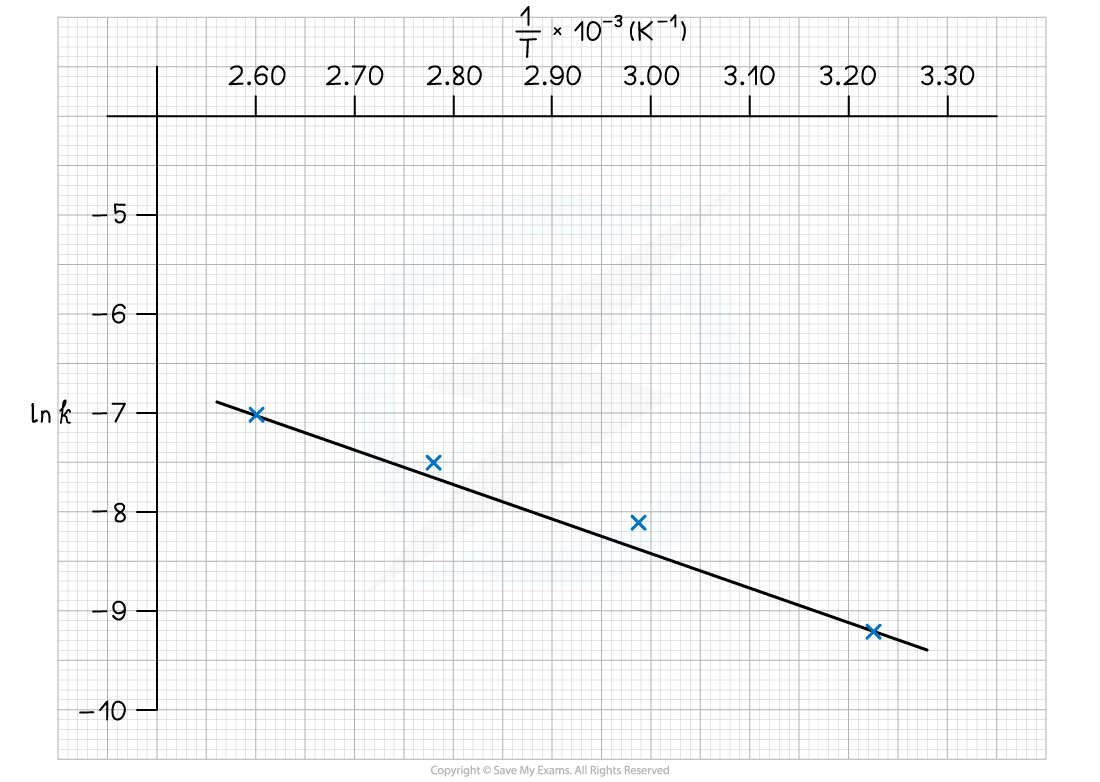
Answer 3: 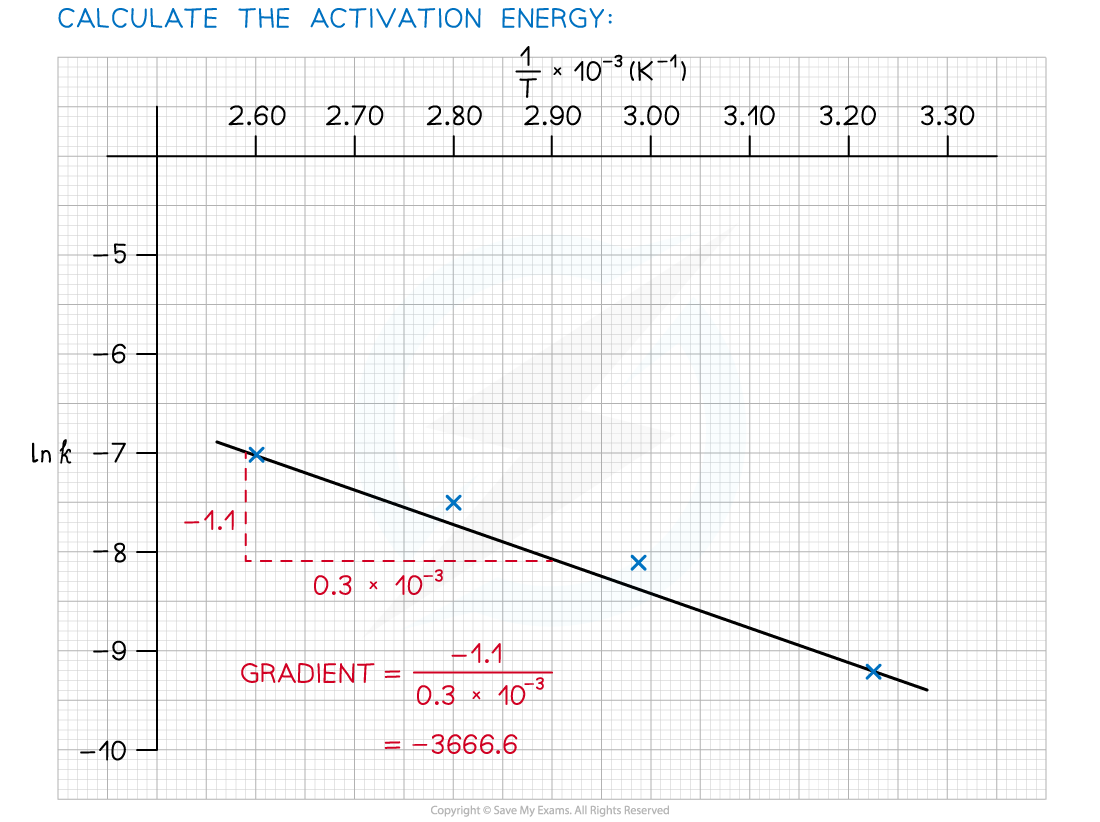
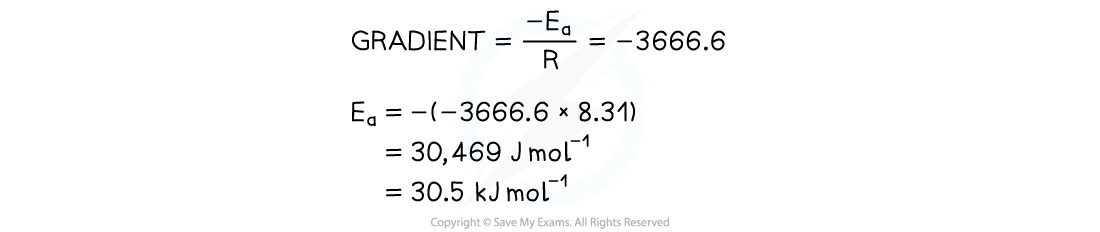
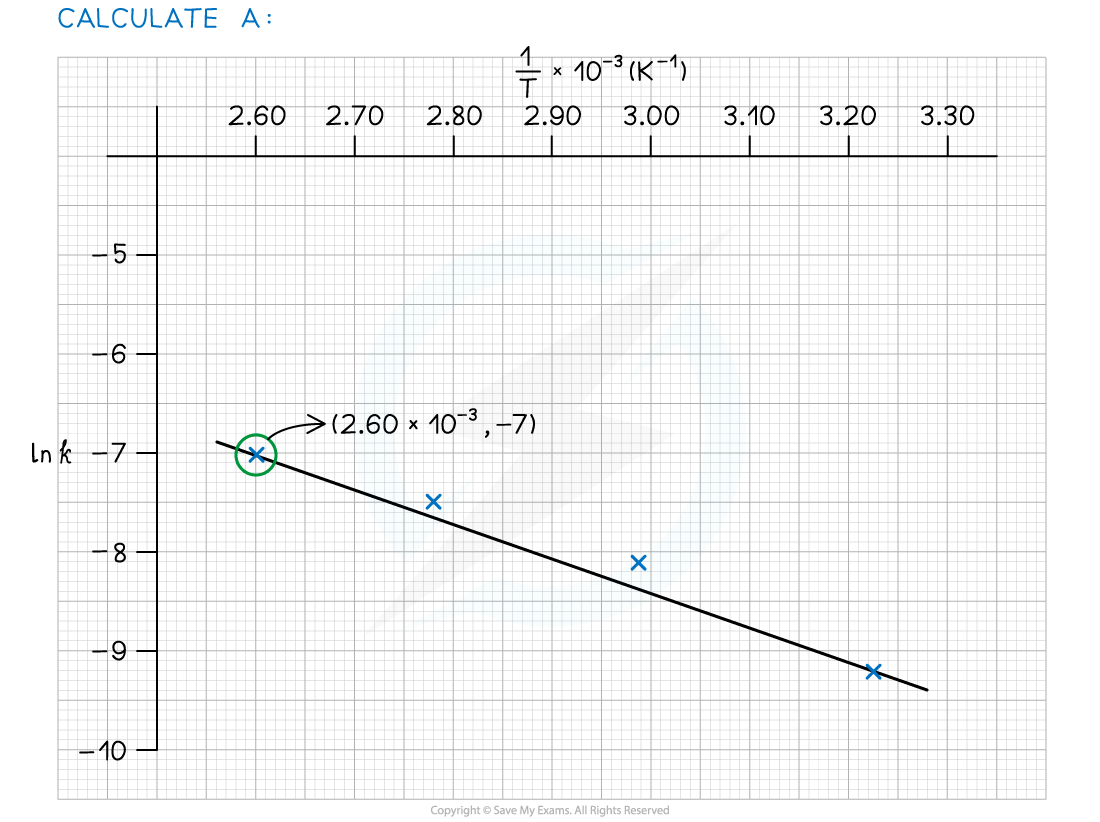
Exam Tip
You are not required to learn these equations.However, you do need to be able to rearrange them, and knowing them is helpful in understanding the effects of temperature on the rate constant.
