Elements & Compounds
- Elements are substances made from one kind of atom
- Compounds are made from two or more elements chemically combined
- Elements take part in chemical reactions in which new substances are made in processes that most often involve an energy change
- In these reactions, atoms combine together in fixed ratios that will give them full outer shells of electrons, producing compounds
- The properties of compounds can be quite different from the elements that form them
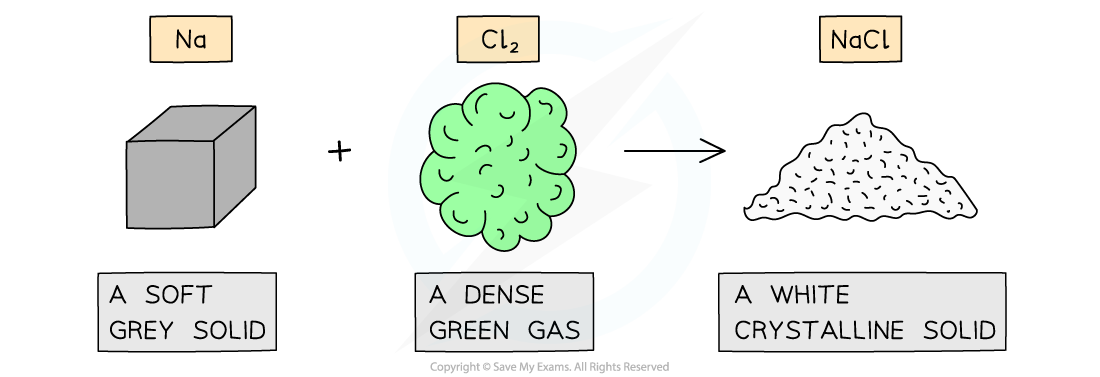
The properties of sodium chloride are quite different from sodium and chlorine
Mixtures
- In a mixture, elements and compounds are interspersed with each other, but are not chemically combined
- This means the components of a mixture retain the same characteristic properties as when they are in their pure form
- So, for example, the gases nitrogen and oxygen when mixed in air, retain the same characteristic properties as they would have if they were separate
- Substances will burn in air because the oxygen present in the air supports combustion

Mixtures at the molecular level
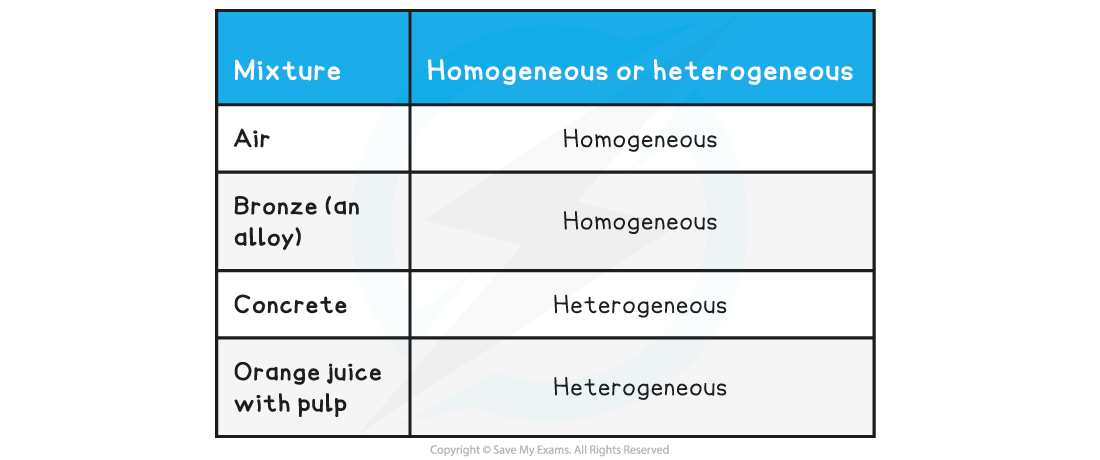
Homogeneous or heterogeneous
- A homogeneous mixture has uniform composition and properties throughout
- A heterogeneous mixture has non-uniform composition, so its properties are not the same throughout
- It is often possible to see the separate components in a heterogeneous mixture, but not in a homogeneous mixture
Types of Mixtures
Separating Mixtures
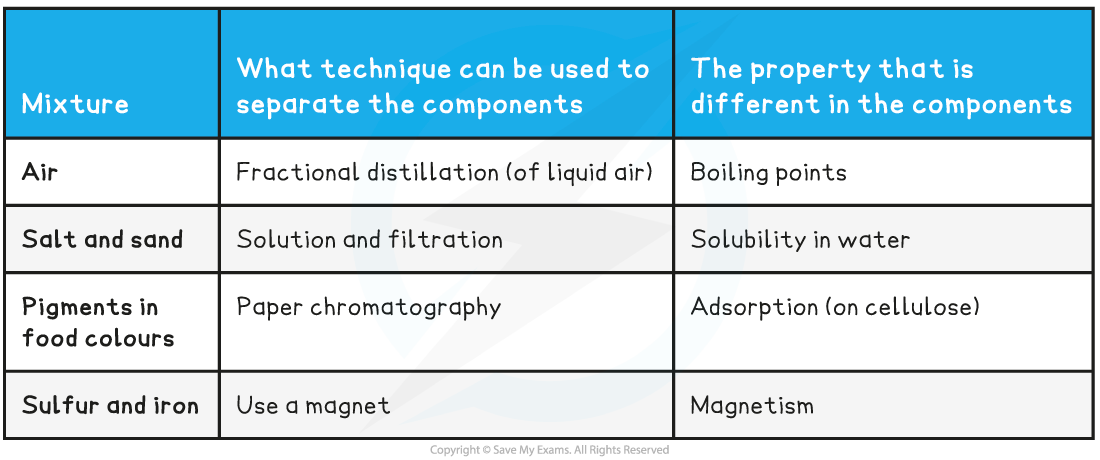
- The components retain their individual properties in a mixture and we can often separate them relatively easily. The technique we choose to achieve this will take advantage of a suitable difference in the physical properties of the components
Mixtures & Separation Techniques