Electronegativity: Definition
- Electronegativity is the ability of an atom to attract a pair of electrons towards itself in a covalent bond
- This phenomenon arises from the positive nucleus’s ability to attract the negatively charged electrons, in the outer shells, towards itself
- The Pauling scale is used to assign a value of electronegativity for each atom
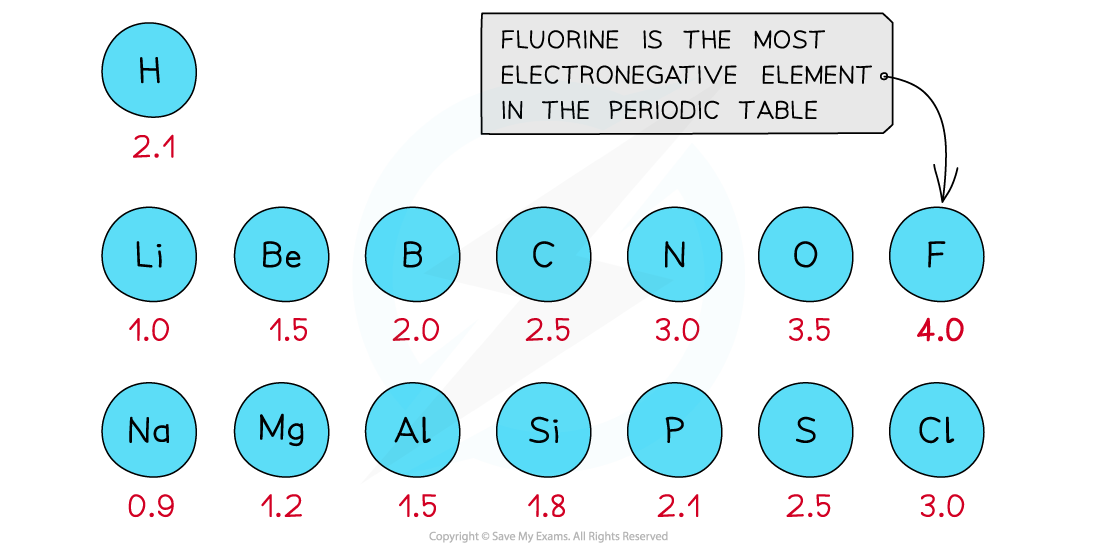
First three rows of the periodic table showing electronegativity values
- Fluorine is the most electronegative atom on the Periodic Table, with a value of 4.0 on the Pauling Scale
- It is best at attracting electron density towards itself when covalently bonded to another atom
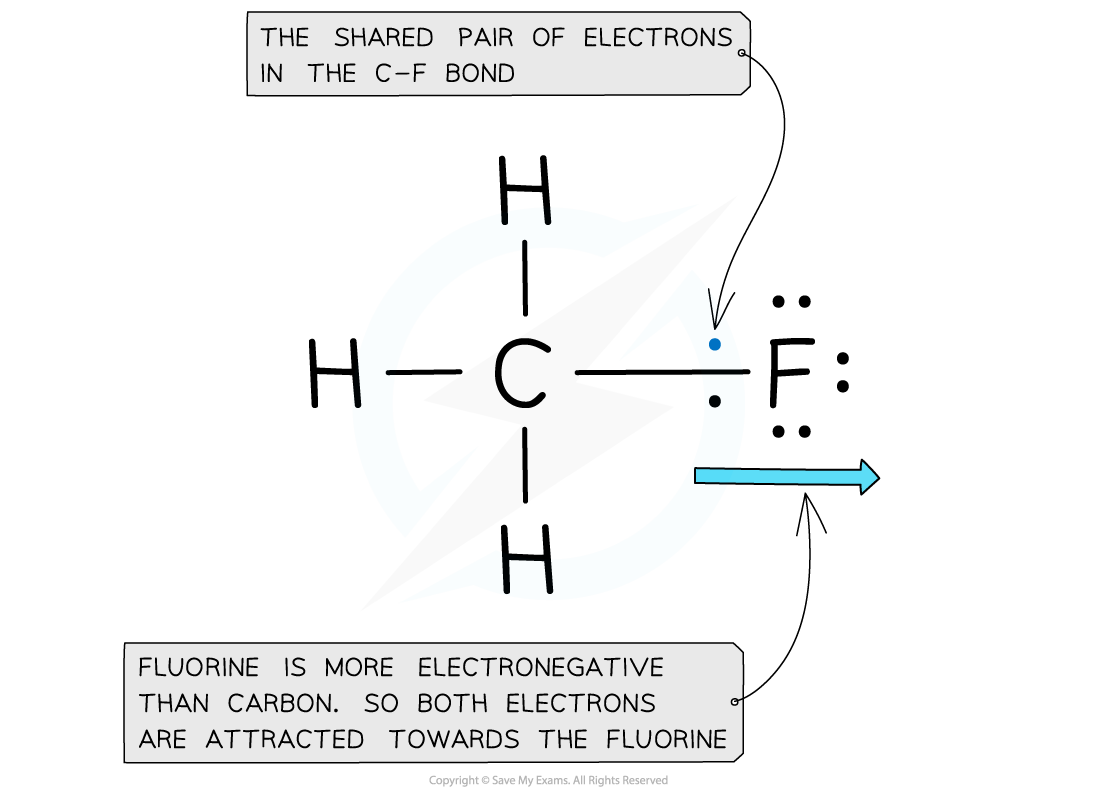
Electron distribution in the C-F bond of fluoromethane
Electronegativity: Affecting Factors
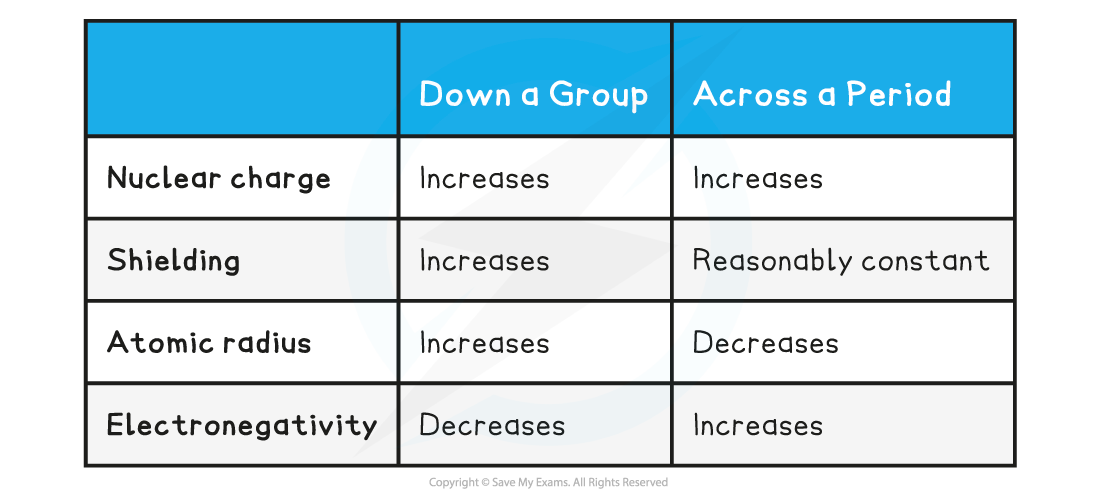
Nuclear charge
- Attraction exists between the positively charged protons in the nucleus and negatively charged electrons found in the energy levels of an atom
- An increase in the number of protons leads to an increase in nuclear attraction for the electrons in the outer shells
- Therefore, an increased nuclear charge results in an increased electronegativity
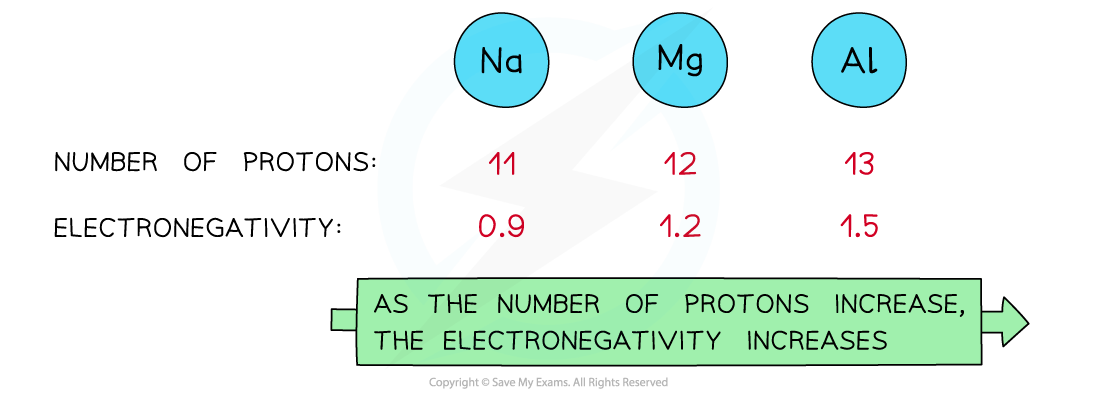
As the nuclear charge increases, the electronegativity of an element increases as well
Atomic radius
- The atomic radius is the distance between the nucleus and electrons in the outermost shell
- Electrons closer to the nucleus are more strongly attracted towards its positive nucleus
- Those electrons further away from the nucleus are less strongly attracted towards the nucleus
- Therefore, an increased atomic radius results in a decreased electronegativity
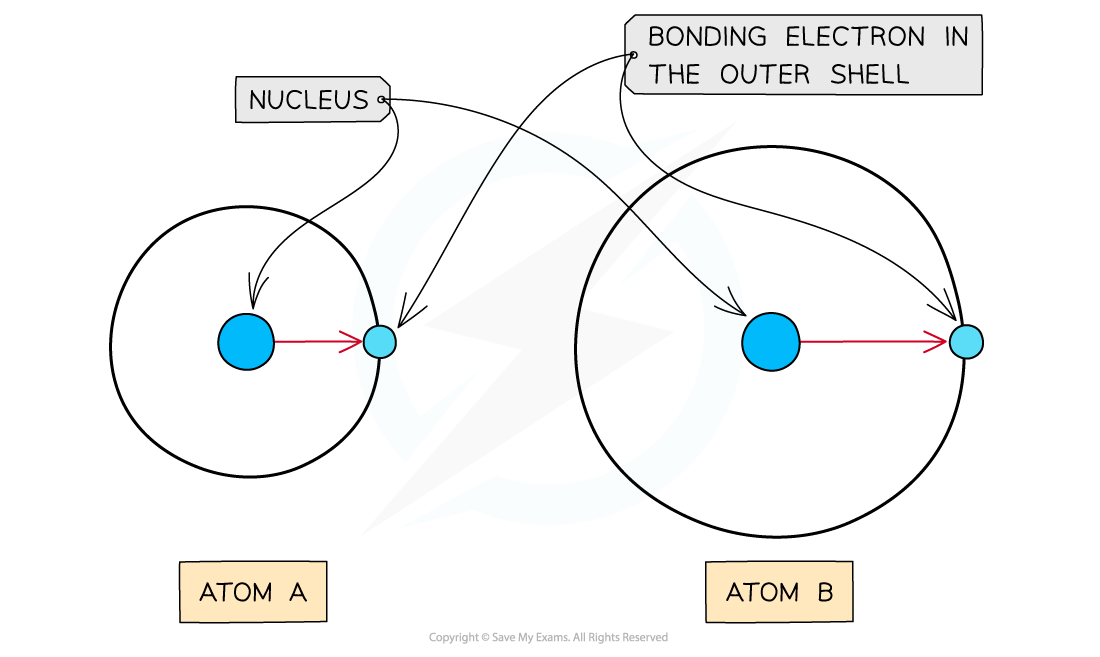
As the atomic radius increases, the nucleus has less of an attraction for the bonding electrons causing atom A to have a higher electronegativity than atom B
Electronegativity: Trends
- Electronegativity varies across periods and down the groups of the periodic table
Down a group
- There is a decrease in electronegativity going down the group
- The nuclear charge increases as more protons are being added to the nucleus
- However, each element has an extra filled electron shell, which increases shielding
- The addition of the extra shells increases the distance between the nucleus and the outer electrons resulting in larger atomic radii
- Overall, there is decrease in attraction between the nucleus and outer bonding electrons
- We say the effective nuclear charge has decreased down the group

Electronegativity decreases going down the groups of the periodic table
Across a period
- Electronegativity increases across a period
- The nuclear charge increases with the addition of protons to the nucleus
- Shielding remains the same across the period as no new shells are being added to the atoms
- The nucleus has an increasingly strong attraction for the bonding pair of electrons of atoms across the period
- This results in smaller atomic radii

Electronegativity increases going across the periods of the periodic table
Trends down a Group & across a Period Table
Exam Tip
Make sure you learn the definition of electronegativity and can distinguish it from electron affinity as the two are often confused. Electronegativity is about chemical character and only applies to considerations of covalent bonds whereas electron affinity is a thermodynamic value that is measurable and applies to the formation of negative ions.You may come across something called electropositivity - this is a term used to describe the character of elements to form positive ions and is useful when talking about metal atoms and metal ions
