DP Chemistry Questionbank

Additional higher level (AHL)
Description
[N/A]Directly related questions
-
16N.1.hl.TZ0.14:
Which species has bond angles of 90°?
A. AlCl4-
B. Cl4-
C. NH4+
D. SiCl4
-
16N.2.hl.TZ0.7b:
(i) Sketch a graph of pH against volume of a strong base added to a weak acid showing how you would determine pKa for the weak acid.
(ii) Explain, using an equation, why the pH increases very little in the buffer region when a small amount of alkali is added.
-
16N.2.hl.TZ0.4j:
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
-
16N.3.hl.TZ0.22c:
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
-
20N.2.hl.TZ0.1d(iii):
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
-
20N.2.hl.TZ0.2g(i):
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
-
20N.2.hl.TZ0.5e:
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
-
17M.1.hl.TZ1.8:
What is the charge on the iron(III) complex ion in [Fe(OH)2(H2O)4]Br?
A. 0
B. 1+
C. 2+
D. 3+
- 17M.1.hl.TZ1.11: Which combination describes the PH4+ ion?
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.hl.TZ1.36: What is the product of the reduction of 2-methylbutanal? A. 2-methylbutan-1-ol B. ...
-
17M.2.hl.TZ1.4a:
Formulate an equation for the oxidation of nickel(II) sulfide to nickel(II) oxide.
-
17M.2.hl.TZ1.4c.iii:
Use your answers to (c)(i) and (c)(ii), to determine the temperature, in °C, at which the decomposition of liquid tetracarbonylnickel to nickel and carbon monoxide becomes favourable.
(If you did not get answers to (c)(i) and (c)(ii), use and respectively but these are not the correct answers.) - 17M.2.hl.TZ1.7d: Explain the mechanism for the nitration of benzene, using curly arrows to show the movement of...
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.hl.TZ1.28a:
Describe what happens to plane-polarized light when it passes through a solution of an optically active compound.
-
17M.2.hl.TZ2.2b.ii:
Calculate the Gibbs free energy, ΔG θ, in kJ, which is released by the corrosion of 1 mole of iron. Use section 1 of the data booklet.
-
17M.2.hl.TZ2.5b.ii:
The following mechanism is proposed for the reaction.
Identify the rate determining step giving your reason.
-
17M.2.hl.TZ2.7a.i:
Deduce what information can be obtained from the 1H NMR spectrum.
-
17M.2.hl.TZ1.5a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17N.1.hl.TZ0.6:
The graph represents the first ten ionisation energies (IE) of an element.
What is the element?
A. O
B. S
C. Ne
D. Cl
-
17N.2.hl.TZ0.4a:
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
- 21M.2.hl.TZ1.3e: The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure...
- 21M.2.hl.TZ1.3g: Transition metals like iron can form complex ions. Discuss the bonding between transition metals...
-
21M.2.hl.TZ1.5e(ii):
Explain why the major organic product is 2-bromopropane and not 1-bromopropane.
-
21M.2.hl.TZ1.7c:
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
-
21M.2.hl.TZ2.3c:
Calculate the cell potential using section 24 of the data booklet.
- 18M.1.hl.TZ1.17: Which statement is correct? A. If ΔH < 0, reaction is always spontaneous. B. If ΔH...
-
18M.1.hl.TZ1.36:
Which molecule contains a chiral carbon?
A. CH3CH2CHBrCH2CH3
B. CH3CH2CHBrCH3
C. CH2BrCH(CH3)CH2Br
D. CH3CH2CH2CH2CH2Br
- 18M.1.hl.TZ1.37: Which reagents are needed to convert nitrobenzene to phenylamine in 2 steps?
-
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?
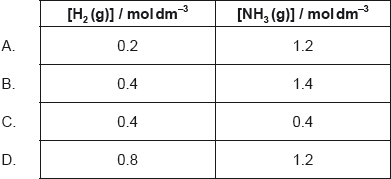
- 18M.1.hl.TZ1.30: Which combination would electroplate an object with copper?
-
18M.2.hl.TZ1.3c.iv:
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
-
18M.2.hl.TZ2.9b.iii:
Draw the structure of the intermediate formed stating its shape.
- 21N.1.hl.TZ0.40: Which substance has the following 1H NMR spectrum? SDBS, National Institute of Advanced...
- 21N.1.hl.TZ0.5: Which statement explains why the second ionization energy of aluminium is higher than the first...
-
21N.2.hl.TZ0.1d:
Predict the number of 1H NMR signals, and splitting pattern of the –CH3 seen for propanone (CH3COCH3) and propanal (CH3CH2CHO).
-
21N.2.hl.TZ0.8:
The standard electrode potential of zinc can be measured using a standard hydrogen electrode (SHE).
Draw and annotate the diagram to show the complete apparatus required to measure the standard electrode potential of zinc.
-
18N.2.hl.TZ0.6c:
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
- 18N.2.hl.TZ0.4c: Sketch a graph to show the relative values of the successive ionization energies of boron.
-
18N.1.hl.TZ0.21:
The rate expression for the reaction is: rate = k [NO]2[O2].
2NO (g) + O2 (g) → 2NO2 (g)
Which mechanism is not consistent with this rate expression?
- 18N.1.hl.TZ0.36: What is the major product of the reaction of HBr with but-1-ene? A. 1-bromobutane B. ...
-
18N.1.hl.TZ0.12:
What is the number of sigma (σ) and pi (π) bonds in the molecule (NC)2C=C(CN)2?
- 18N.1.hl.TZ0.5: The values for the first three successive ionization energies for two elements X and Z are...
- 18N.2.hl.TZ0.6e.i: State a suitable reagent for the reduction of butanoic acid.
-
22M.1.hl.TZ1.38:
Which compound produces the following 1H NMR spectrum?
[Spectral Database for Organic Compounds, SDBS. SDBS Compounds and Spectral Search. [graph] Available at:
https://sdbs.db.aist.go.jp [Accessed 3 January 2019].]
A. propanalB. propanone
C. propane
D. methlypropane
- 22M.1.hl.TZ1.27: In which set are the salts arranged in order of increasing pH? A. HCOONH4 < KBr < NH4Br...
- 22M.1.hl.TZ1.35: What are the type of reaction and role of the nitronium ion, NO2+, in the following...
- 22M.1.hl.TZ1.36: What is molecule Z that is formed in step 1 of this synthetic route?
- 22M.1.hl.TZ2.26: A weak base is titrated with a strong acid. Which value of pKb can be estimated from this...
- 22M.1.hl.TZ2.32: Which sequence of reagents converts propene to propanone?
-
22M.2.hl.TZ1.2b(i):
Calculate the standard potential, in V, of a cell formed by magnesium and steel half-cells. Use section 24 of the data booklet and assume steel has the standard electrode potential of iron.
-
22M.2.hl.TZ1.3c(iii):
Calculate the entropy change for the Haber–Bosch process, in J mol–1 K–1 at 298 K. Use your answer to (b)(i) and section 1 of the data booklet.
-
22M.2.hl.TZ1.4e:
Mg(OH)+ is a complex ion, but Mg is not regarded as a transition metal. Contrast Mg with manganese, Mn, in terms of one characteristic chemical property of transition metals, other than complex ion formation.
-
22M.2.hl.TZ1.4c(ii):
Calculate the concentration, in mol dm–3, of ammonia molecules in the solution with pH = 9.3. Use section 21 of the data booklet.
-
22M.2.hl.TZ2.3a(i):
Iron(II) is oxidized by bromine.
2Fe2+ (aq) + Br2 (l) 2Fe3+ (aq) + 2Br− (aq)
Calculate the E⦵cell, in V, for the reaction using section 24 of the data booklet.
-
22M.2.hl.TZ2.4d(iii):
Calculate the Gibbs free energy change, ΔG⦵, in kJ mol−1, for the reaction at 298 K. Use section 1 of the data booklet.
-
22M.2.hl.TZ2.7b(ii):
Identify the number of sigma and pi bonds in HCN.
-
22M.2.hl.TZ2.8e(i):
Explain the mechanism of the reaction between 1-bromopropane, CH3CH2CH2Br, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
-
22M.2.hl.TZ1.4c(iii):
An aqueous solution containing high concentrations of both NH3 and NH4+ acts as an acid-base buffer solution as a result of the equilibrium:
NH3 (aq) + H+ (aq) NH4+ (aq)
Referring to this equilibrium, outline why adding a small volume of strong acid would leave the pH of the buffer solution almost unchanged.
-
19M.2.hl.TZ1.2e:
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
-
19M.2.hl.TZ1.1e:
The organic product is not optically active. Discuss whether or not the organic product is a racemic mixture.
-
19M.2.hl.TZ1.5d(ii):
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
-
19M.2.hl.TZ1.7:
An aqueous solution of silver nitrate, AgNO3 (aq), can be electrolysed using platinum electrodes.
Formulate the half-equations for the reaction at each electrode during electrolysis.
Cathode (negative electrode):
Anode (positive electrode):
-
19M.2.hl.TZ2.3d(i):
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
-
19M.1.hl.TZ1.17:
Which equation represents the standard enthalpy of atomization of bromine, Br2?
A. Br2 (l) → Br (g)
B. Br2 (l) → 2Br (g)
C. Br2 (l) → 2Br (l)
D. Br2 (l) → Br (l)
-
19M.1.hl.TZ2.27:
The following equation represents the dissociation of water at 25 °C.
2H2O (l) H3O+ (aq) + OH− (aq) ΔH = +56 kJ
Which changes occur as the temperature increases?
A. [H3O+] increases and pH will decrease.
B. [H3O+] decreases and pH will increase.
C. [H3O+] increases and pH will increase.
D. [H3O+] decreases and pH will decrease.
-
19M.2.hl.TZ1.4b(ii):
Two more trials (2 and 3) were carried out. The results are given below.
Determine the rate equation for the reaction and its overall order, using your answer from (b)(i).
Rate equation:
Overall order:
-
19N.2.hl.TZ0.3b(i):
Determine the rate expression from the results, explaining your method.
- 19N.2.hl.TZ0.3a(iii): Outline why it is the major product.
-
19N.1.hl.TZ0.40:
Which is the 1H NMR spectrum of tetramethylsilane, TMS, (CH3)4Si?
- 19N.1.hl.TZ0.5: Which shows the first ionization energies of successive elements across period 2, from left to...
-
19N.2.hl.TZ0.6c(iv):
Deduce the half-equation for the formation of the gas identified in (c)(iii).
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 16N.2.hl.TZ0.6c: Suggest why the rate of alkaline hydrolysis of an enantiomer of iodopropane is greater than that...
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
16N.2.hl.TZ0.5b:
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
-
20N.1.hl.TZ0.40:
Which compound with the molecular formula has this high resolution ?
From: libretexts.org. Courtesy of Chris Schaller, Professor (Chemistry)
at College of Saint Benedict/Saint John’s University.A. but-3-en-2-ol,
B. butanal,
C. butanone,
D. but-3-en-1-ol,
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
20N.1.hl.TZ0.36:
What will be the major product in the reaction between but-1-ene and ?
A. 2-bromobut-1-ene
B. 1-bromobut-1-ene
C. 2-bromobutane
D. 1-bromobutane
-
20N.1.hl.TZ0.31:
Which statement is correct when a zinc spoon is electroplated with silver?
A. The cathode (negative electrode) is made of silver.
B. The anode (positive electrode) is the zinc spoon.
C. The anode (positive electrode) is made of silver.
D. The electrolyte is zinc sulfate solution.
-
20N.1.hl.TZ0.8:
Which of these statements are correct?
I. Zinc is not a transition element.
II. Ligands are Lewis bases.
III. Manganese(II) chloride is paramagnetic.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
20N.2.hl.TZ0.4d(iii):
Calculate the standard free energy change, , in , for the cell using sections 1 and 2 of the data booklet.
-
20N.3.hl.TZ0.13c:
Predict, giving a reason, how an increase in temperature affects the potential of this cell.
-
17M.1.hl.TZ1.20:
The table gives rate data for the reaction in a suitable solvent.
C4H9Br + OH− → C4H9OH + Br−
Which statement is correct?
A. The rate expression is rate = k [C4H9Br] [OH− ].
B. The rate increases by a factor of 4 when the [OH− ] is doubled.
C. C4H9Br is a primary halogenoalkane.
D. The reaction occurs via SN1 mechanism.
-
17M.2.hl.TZ1.1b:
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
-
17M.2.hl.TZ1.1c:
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
-
17M.2.hl.TZ1.3b.i:
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
-
17M.2.hl.TZ1.3c.ii:
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
-
17M.2.hl.TZ1.4c.ii:
Calculate a value for in kJ.
-
17M.1.hl.TZ2.16:
The Born-Haber cycle for potassium oxide is shown below:
Which expression represents the lattice enthalpy in kJ mol–1?
A. –361 + 428 + 838 + 612
B. –(–361) + 428 + 838 + 612
C. –361 + 428 + 838 – 612
D. –(–361) + 428 + 838 – 612
-
17M.2.hl.TZ2.3b:
Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
-
17N.1.hl.TZ0.17:
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
- 17N.2.hl.TZ0.3d.ii: Deduce the charge on the complex ion and the oxidation state of cobalt.
-
17N.2.hl.TZ0.8e:
Explain the mechanism of the reaction between 2-bromo-2-methylpropane, (CH3)3CBr, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
- 17N.1.hl.TZ0.13: What is the hybridization state and electron domain geometry around the circled C, N and...
-
21M.1.hl.TZ1.16:
The table shows the variation of standard Gibbs energy with temperature for a reversible reaction.
What can be concluded about the reaction?
A. Equilibrium shifts left as temperature increases.
B. The forward reaction is more spontaneous below 300 K.
C. Entropy is higher in the products than in the reactants.
D. Kc decreases as temperature increases.
-
21M.1.hl.TZ2.30:
What would be the electrode potential, E⦵, of the Mn2+ (aq)|Mn (s) half-cell if Fe3+ (aq)|Fe2+ (aq) is used as the reference standard?
Mn2+ (aq) + 2e− Mn (s) E⦵ = −1.18 V
Fe3+ (aq) + e− Fe2+ (aq) E⦵ = +0.77 VA. −1.95 V
B. −0.41 V
C. +0.41 V
D. +1.95 V
-
21M.2.hl.TZ2.2a(ii):
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
-
18M.1.hl.TZ2.36:
Propene is reacted first with hydrogen chloride to produce X which is then reacted with aqueous sodium hydroxide to give Y. Finally, Y is reacted with excess acidified potassium dichromate solution.
What is the major product, Z?
A. CH3CH(OH)CH3
B. CH3COCH3
C. CH3CH2CHO
D. CH3(CH2)2COOH
-
18M.1.hl.TZ2.13:
Which overlap of atomic orbitals leads to the formation of only a sigma (σ) bond?
I. s − p
II. p − p
III. s − s
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
18M.2.hl.TZ1.2g.i:
Describe how sigma (σ) and pi () bonds are formed.
- 18M.1.hl.TZ1.40: Which would be the most effective method to distinguish between liquid propan-1-ol and...
- 18M.1.hl.TZ1.27: Which combination of acid and base is most likely to have a pH of 8.5 at the equivalence point in...
-
18M.1.hl.TZ2.30:
Two cells undergoing electrolysis are connected in series.
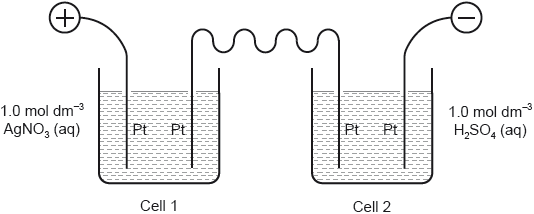
If g of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
Ar(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
A.
B.
C.
D.
-
18M.2.hl.TZ1.1l.iii:
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
-
18M.2.hl.TZ1.7b:
Outline why the rate of reaction of the similar bromo-compounds is faster.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
-
21N.1.hl.TZ0.21:
The rate equation for a reaction is:
rate = k[A][B]
Which mechanism is consistent with this rate equation?
A. 2A I Fast
I + B → P SlowB. A + B I Fast
I + A → P SlowC. A → I Slow
I + B → P FastD. B I Fast
I + A → P Slow - 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
-
18N.2.hl.TZ0.6b.ii:
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
- 18N.2.hl.TZ0.9c: Explain the mechanism of the reaction between 1-bromopropane with aqueous sodium hydroxide using...
- 18N.1.hl.TZ0.20: Compounds X and Y were mixed and the time taken for a colour to appear was recorded at various...
- 18N.2.hl.TZ0.8c: Predict the chemical shift and splitting pattern of the signal produced by the hydrogen atoms...
- 22M.1.hl.TZ1.26: Which statement explains the Lewis acid–base nature of the chloride ion in this reaction? C2H5+...
-
22M.1.hl.TZ2.30:
Which E⦵ value, in V, for the reaction Mn (s) + Zn2+ (aq) → Mn2+ (aq) + Zn (s) can be deduced from the following equations?
Mn (s) + 2Ag+ (aq) → Mn2+ (aq) + 2Ag (s) E⦵ = 1.98 V
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) E⦵ = 1.10 V
Cu (s) + 2Ag+ (aq) → Cu2+ (aq) + 2Ag (s) E⦵ = 0.46 V
A. 0.42
B. 1.34
C. 2.62
D. 3.54
- 22M.1.hl.TZ2.37: What is the product of the reaction of propanal with lithium aluminium hydride, LiAlH4? A. ...
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
- 22M.2.hl.TZ1.3c(iv): Outline, with reference to the reaction equation, why this sign for the entropy change is expected.
-
22M.2.hl.TZ1.2c(ii):
The reaction is first order with respect to HCl. Calculate the time taken, in seconds (s), for half of the Mg to dissolve when [HCl] = 0.5 mol dm–3.
- 22M.2.hl.TZ1.6a(iv): State a technique used to determine the length of the bonds between N and O in solid HNO3.
-
22M.2.hl.TZ2.7a(ii):
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
- 22M.2.hl.TZ2.7b(iii): State the hybridization of the carbon atom in HCN.
- 22M.1.hl.TZ2.27: Which species are both Lewis and Brønsted–Lowry bases? I. CN−II. OH−III. NH3 A. I and II...
-
19M.2.hl.TZ2.5c:
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
-
19M.2.hl.TZ2.6d(i):
Outline why the major product, C6H5–CHBr–CH3, can exist in two forms and state the relationship between these forms.
Two forms:
Relationship:
-
19M.2.hl.TZ2.4e(iii):
Predict, giving a reason, whether the reduction of ReO4− to [Re(OH)2]2+ would oxidize Fe2+ to Fe3+ in aqueous solution. Use section 24 of the data booklet.
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.2.hl.TZ2.6e:
The minor product, C6H5–CH2–CH2Br, can be directly converted to an intermediate compound, X, which can then be directly converted to the acid C6H5–CH2–COOH.
C6H5–CH2–CH2Br → X → C6H5–CH2–COOH
Identify X.
- 19M.1.hl.TZ1.21: Which is correct for the reaction mechanism shown?
-
19M.1.hl.TZ1.31:
Which is not a requirement of the standard hydrogen electrode (SHE)?
A. V = 1 dm3
B. p(H2) = 100 kPa
C. use of platinum as the electrode material
D. [H3O+] = 1 mol dm−3
- 19M.1.hl.TZ1.30: Which factors affect the amount of product formed at the cathode during electrolysis of molten...
- 19M.1.hl.TZ1.26: Which is a Lewis acid but not a Brønsted−Lowry acid? A. AlCl3 B. CH3CO2H C. HF D. CCl4
-
19M.1.hl.TZ2.30:
Consider the following table of standard electrode potentials.
Which is the strongest oxidizing agent?
A. Pb2+
B. Pb
C. Al3+
D. Al
- 19M.1.hl.TZ1.20: Which graph is obtained from a first order reaction?
- 19M.1.hl.TZ1.12: Which species has delocalized electrons? A. OH− B. H2CO C. CO2 D. CO32−
- 19M.1.hl.TZ2.36: Which compound exists as two configurational isomers? A. CBr2=CH2 B. CH2=CHBr C....
- 19N.2.hl.TZ0.1f: Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor...
-
19N.1.hl.TZ0.22:
What is the intercept on the y-axis when a graph of lnk is plotted against on the x-axis?
A. lnA
B.
C.
D.
- 19N.2.hl.TZ0.5b(i): Identify the most suitable indicator for the titration using section 22 of the data booklet.
- 19N.2.hl.TZ0.6e(v): Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the...
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
-
16N.2.hl.TZ0.7a:
Calculate the pH of 0.0100 mol dm–3 methanoic acid stating any assumption you make. Ka = 1.6 × 10–4.
-
16N.2.hl.TZ0.4d:
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
-
16N.2.hl.TZ0.4k:
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
-
20N.1.hl.TZ0.17:
Which reaction becomes more spontaneous as temperature increases?
A.
B.
C.
D.
-
20N.2.hl.TZ0.2g(ii):
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
-
17M.1.hl.TZ1.16:
Which equation represents enthalpy of hydration?
A. Na(g) → Na+(aq) + e−
B. Na+(g) → Na+(aq)
C. NaCl(s) → Na+(g) + Cl−(g)
D. NaCl(s) → Na+(aq) + Cl−(aq)
-
17M.2.hl.TZ1.3b.ii:
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
-
17M.2.hl.TZ1.4b:
The nickel obtained from another ore, nickeliferous limonite, is contaminated with iron. Both nickel and iron react with carbon monoxide gas to form gaseous complexes, tetracarbonylnickel, , and pentacarbonyliron, . Suggest why the nickel can be separated from the iron successfully using carbon monoxide.
-
17M.2.hl.TZ1.5d.i:
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
- 17M.1.hl.TZ2.31: What are the relative volumes of gas given off at E and F during electrolysis of the two cells...
- 17M.1.hl.TZ2.35: Which pair of isomers always shows optical activity? A. Cis-trans B. Enantiomers C. ...
-
17M.2.hl.TZ2.4b.i:
Discuss the bonding in the resonance structures of ozone.
-
17M.2.hl.TZ2.4b.ii:
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
-
17M.2.hl.TZ2.5b.i:
State the rate expression for the reaction.
-
17M.2.hl.TZ2.9b.ii:
The standard enthalpy change, ΔH θ, for the hydrogenation of propene is –124.4 kJ mol–1. Predict the temperature above which the hydrogenation reaction is not spontaneous.
- 17N.1.hl.TZ0.33: Propene reacts separately with H2O/H+ and H2/Ni to give products X and Z respectively. What...
-
17N.2.hl.TZ0.5b:
Calculate the standard entropy change for this reaction using the following data.
- 17N.2.hl.TZ0.8a.v: Draw three-dimensional representations of the two enantiomers.
-
17N.1.hl.TZ0.18:
Which equation represents the lattice enthalpy of magnesium sulfide?
A. MgS (s) → Mg (g) + S (g)
B. MgS (s) → Mg+ (g) + S– (g)
C. MgS (s) → Mg2+ (g) + S2– (g)
D. MgS (s) → Mg (s) + S (s)
-
17N.2.hl.TZ0.8c:
State the reagents used in the nitration of benzene.
- 21M.1.hl.TZ2.26: Which is correct? A. Electrophiles are Brønsted–Lowry acids. B. Nucleophiles are...
- 21M.1.hl.TZ2.35: Which compound shows cis-trans isomerism? A. CH3CH=CCl2 B. CCl2=CH2 C. D.
-
21M.2.hl.TZ1.8b:
Sketch the general shape of the variation of pH when 50 cm3 of 0.001 mol dm–3 NaOH (aq) is gradually added to 25 cm3 of 0.001 mol dm–3 CH3CH2COOH (aq).
-
21M.2.hl.TZ2.1b(ii):
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
- 21M.2.hl.TZ2.4c: State the hybridization of the carbon I and II atoms in but-2-ene.
- 21M.2.hl.TZ2.4h(i): Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
- 21M.2.hl.TZ2.4h(ii): Outline how two enantiomers can be distinguished using a polarimeter.
-
21M.2.hl.TZ2.6b:
Determine the value and unit of the rate constant using the rate expression in (a).
- 18M.1.hl.TZ1.12: Which molecules have at least one sp2 hybridized atom? I. CH3COOH II. ...
-
18M.2.hl.TZ1.1h:
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
- 18M.1.hl.TZ1.20: The reaction between NO2 and F2 gives the following rate data at a certain temperature. What...
-
18M.1.hl.TZ2.16:
Which value represents the lattice enthalpy, in kJ mol−1, of strontium chloride, SrCl2?
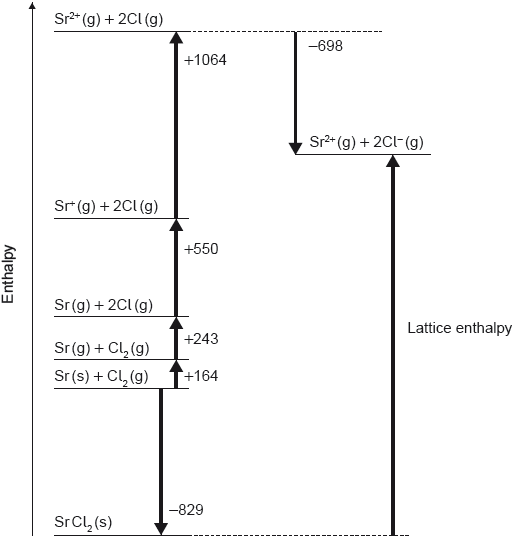
A. – (–829) + 164 + 243 + 550 + 1064 – (–698)
B. –829 + 164 + 243 + 550 + 1064 – 698
C. – (–829) + 164 + 243 + 550 + 1064 – 698
D. –829 + 164 + 243 + 550 + 1064 – (–698)
-
18M.2.hl.TZ1.5e:
Explain, using appropriate equations, how a suitably concentrated solution formed by the partial neutralization of 2,2-dimethylpropanoic acid with sodium hydroxide acts as a buffer solution.
-
18M.2.hl.TZ2.3a.iii:
Hydrogen spectral data give the frequency of 3.28 × 1015 s−1 for its convergence limit.
Calculate the ionization energy, in J, for a single atom of hydrogen using sections 1 and 2 of the data booklet.
-
18M.2.hl.TZ2.7d:
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
-
18M.2.hl.TZ2.3c.v:
Deduce the gas formed at the anode (positive electrode) when graphite is used in place of copper.
-
18M.2.hl.TZ2.7c.ii:
Deduce, giving a reason, the more likely structure.
-
18M.2.hl.TZ2.9c:
Nitrobenzene, C6H5NO2, can be converted to phenylamine via a two-stage reaction.
In the first stage, nitrobenzene is reduced with tin in an acidic solution to form an intermediate ion and tin(II) ions. In the second stage, the intermediate ion is converted to phenylamine in the presence of hydroxide ions.
Formulate the equation for each stage of the reaction.
- 21N.1.hl.TZ0.37: Which attacking species is matched with its mechanism of reaction?
- 21N.1.hl.TZ0.20: Which graph shows a first order reaction?
-
21N.2.hl.TZ0.2b:
Sodium emits yellow light with a frequency of 5.09 × 1014 Hz when electrons transition from 3p to 3s orbitals.
Calculate the energy difference, in J, between these two orbitals using sections 1 and 2 of the data booklet.
Darling, D, n.d. D lines (of sodium). [online] Available at <https://www.daviddarling.info/encyclopedia/D/D_lines.html> [Accessed 6 May 2020].
-
21N.2.hl.TZ0.3c(iii):
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
- 21N.2.hl.TZ0.10b(iv): State, giving a reason, if the product of this reaction exhibits stereoisomerism.
-
18N.2.hl.TZ0.6e.ii:
Deduce the product of the complete reduction reaction in (e)(i).
-
18N.1.hl.TZ0.37:
How many chiral carbon atoms are present in one molecule of (CH3)2CHCHClCHBrCH3?
A. 0
B. 1
C. 2
D. 3
- 18N.1.hl.TZ0.40: Which technique may be used to find the bond lengths and bond angles within a molecule? A. ...
- 18N.1.hl.TZ0.25: What is the order of increasing pH for the following solutions of the same concentration? A. ...
- 18N.2.hl.TZ0.5b: Predict, giving your reason, the sign of the standard entropy change of the forward reaction.
- 18N.2.hl.TZ0.3b.ii: Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
- 22M.1.hl.TZ1.12: In which compound are all carbon atoms sp3 hybridized? A. C2H2 B. C2H2Cl2 C. C2Cl4 D. C2Cl6
-
22M.1.hl.TZ1.21:
What is the activation energy according to the following plot of the linear form of the Arrhenius equation?
Arrhenius equation: .
A.
B.
C.
D.
-
22M.1.hl.TZ2.11:
What is the formal charge of the oxygen atom in H3O+?
A. −2
B. −1
C. 0
D. +1
-
22M.1.hl.TZ2.8:
[Cr(OH2)6]3+ is violet and [Cr(NH3)6]3+ is yellow. What is correct?
The Colour Wheel
- 22M.1.hl.TZ2.31: What is the order of increasing mass deposited by this electrolytic cell? Ar Ag = 108, Cu =...
-
22M.2.hl.TZ1.5d(iii):
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
- 22M.2.hl.TZ1.5a(iv): Deduce the hybridization of the central carbon atom in Compound A.
- 22M.2.hl.TZ2.7a(iii): Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer...
-
22M.2.hl.TZ2.8e(ii):
Deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane.
-
19M.2.hl.TZ1.1c(i):
Write the equation for the production of the active nitrating agent from concentrated sulfuric and nitric acids.
-
19M.2.hl.TZ1.3d(ii):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.) -
19M.2.hl.TZ1.5d(iii):
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
-
19M.2.hl.TZ2.2c(ii):
It has been suggested that the reaction occurs as a two-step process:
Step 1: N2O (g) → N2 (g) + O (g)
Step 2: N2O (g) + O (g) → N2 (g) + O2 (g)
Explain how this could support the observed rate expression.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
- 19M.1.hl.TZ1.35: Which solvent is aprotic? A. H2O B. C6H5CH3 C. CH3OH D. CH3NH2
-
19M.1.hl.TZ1.27:
Which has the strongest conjugate base?
A. HCOOH (Ka = 1.8 × 10−4)
B. HNO2 (Ka = 7.2 × 10−4)
C. HCN (Ka = 6.2 × 10−10)
D. HIO3 (Ka = 1.7 × 10−1)
- 19M.1.hl.TZ2.11: Which species has a square planar molecular geometry? A. SF4 B. XeF4 C. CF4 D. PF4+
-
19M.1.hl.TZ2.26:
Where is the buffer region for the titration of a weak acid with a strong base?
- 19M.1.hl.TZ2.6: How is colour produced in transition metal complexes? A. Light is absorbed when electrons are...
- 19N.2.hl.TZ0.1e(i): Identify the steps which absorb ultraviolet light.
-
19N.2.hl.TZ0.6e(vi):
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
- 19N.2.hl.TZ0.6e(ii): Comment on the spontaneity of the disproportionation reaction at 298 K.
-
19N.1.hl.TZ0.38:
Which can show optical activity?
A. CHBrCHCl
B. CH3CH2CHBrCH2CH3
C. (CH3)2CBrCl
D. CH3CH2CH(CH3)Br
-
19N.1.hl.TZ0.36:
In which compound is the halogen substituted the most rapidly by aqueous hydroxide ions?
A. (CH3)3CCl
B. (CH3)3CI
C. CH3CH2CH2CH2Cl
D. CH3CH2CH2CH2I
- 16N.1.hl.TZ0.36: Which is correct for the conversion of propanal to propyl methanoate?
- 16N.1.hl.TZ0.32: Which signs for both Eθcell and ΔGθ result in a spontaneous redox reaction occurring under...
-
16N.1.hl.TZ0.22:
Decomposition of hydrogen peroxide in an aqueous solution proceeds as follows.
2H2O2(aq) → 2H2O(l) + O2(g)
The rate expression for the reaction was found to be: rate = k [H2O2].
Which graph is consistent with the given rate expression?
- 16N.2.hl.TZ0.6b: When one enantiomer undergoes substitution by alkaline hydrolysis approximately 75 % of the...
- 16N.2.hl.TZ0.1f: Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
-
20N.2.hl.TZ0.2f(ii):
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
-
20N.2.hl.TZ0.3d:
Calculate for the reaction in , using section 12 of the data booklet.
The standard molar entropy for oxygen gas is .
-
20N.3.hl.TZ0.13a:
Write the balanced equation for the reaction in this voltaic cell.
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ1.27:
A buffer is produced by mixing 20.0 cm3 of 0.10 mol dm−3 ethanoic acid, CH3COOH(aq), with 0.10 mol dm−3 sodium hydroxide, NaOH(aq).
What is the volume of NaOH required and the pH of the buffer?
-
17M.1.hl.TZ1.31:
In the electrolysis of aqueous potassium nitrate, KNO3(aq), using inert electrodes, 0.1 mol of a gas was formed at the cathode (negative electrode).
Which is correct?
-
17M.2.hl.TZ1.4c.i:
Calculate the standard entropy change, , of the reaction, in , using the values given.
-
17M.2.hl.TZ1.6c.iii:
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
-
17M.2.hl.TZ1.7c:
State the reagents used to convert benzene to nitrobenzene and the formula of the electrophile formed.
- 17M.1.hl.TZ2.26: Which type of bond is formed when a Lewis acid reacts with a Lewis base? A. Covalent B. ...
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.7a.iii:
Suggest the structural formula of this compound.
-
17M.3.hl.TZ2.21c.ii:
Predict the chemical shift and the splitting pattern seen for the hydrogens on the carbon atom circled in the diagram. Use section 27 of the data booklet.
- 17N.2.hl.TZ0.4c: State the type of hybridization shown by the phosphorus atom in PF3.
-
17N.2.hl.TZ0.7c:
Calculate the cell potential, in V, when the standard iodine and manganese half-cells are connected.
-
17N.1.hl.TZ0.31:
What are the products when an aqueous solution of copper(II) sulfate is electrolysed using inert graphite electrodes?
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
- 21M.1.sl.TZ1.19: Which is amphiprotic? A. NH4+ B. PO43− C. H2O D. H3O+
- 21M.1.hl.TZ1.21: Which graphs show a first order reaction? A. V and X B. V and Y C. W and X D. W and Y
-
21M.1.hl.TZ1.40:
Which compound produces the following 1H NMR spectrum?
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
A. PropaneB. Propanone
C. Propanal
D. 2,2-dimethylpropane
-
21M.1.hl.TZ2.17:
Which change results in the largest negative value of ΔS?
A. C2H5OH (l) + SOCl2 (l) → C2H5Cl (l) + SO2 (g) + HCl (g)
B. CaCO3 (s) → CaO (s) + CO2 (g)
C. H2O (l) → H2O (s)
D. NH3 (g) + HCl (g) → NH4Cl (s)
- 21M.1.hl.TZ2.5: The first eight successive ionization energies for an element are shown. In which group is the...
-
21M.2.hl.TZ1.3d:
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
- 21M.2.hl.TZ2.5d(i): Sketch the titration curve of methanoic acid with sodium hydroxide, showing how you would...
-
21M.2.hl.TZ2.5e:
Determine the concentration of methanoic acid in a solution of pH = 4.12. Use section 21 of the data booklet.
-
21M.2.hl.TZ2.5f:
Identify if aqueous solutions of the following salts are acidic, basic, or neutral.
-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
- 18M.1.hl.TZ1.5: Which transition on the diagram corresponds to the ionization of hydrogen in the ground state?
-
18M.2.hl.TZ1.2g.ii:
Deduce the number of σ and bonds in a molecule of ethyne.
-
18M.2.hl.TZ2.5d:
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
-
18M.2.hl.TZ1.7c.ii:
Suggest how this product could be synthesized in one step from butanoic acid.
-
18M.2.hl.TZ2.2d.i:
The graph represents the titration of 25.00 cm3 of 0.100 mol dm−3 aqueous ethanoic acid with 0.100 mol dm−3 aqueous sodium hydroxide.
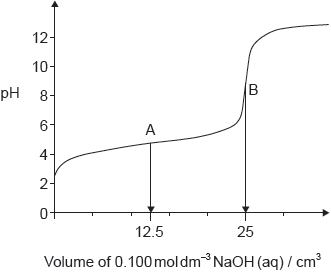
Deduce the major species, other than water and sodium ions, present at points A and B during the titration.
-
18M.2.hl.TZ2.6d:
The rate constant for a reaction doubles when the temperature is increased from 25.0 °C to 35 °C.
Calculate the activation energy, Ea, in kJ mol−1 for the reaction using section 1 and 2 of the data booklet.
-
18M.2.hl.TZ2.9a.ii:
Mass spectra A and B of the two isomers are given.
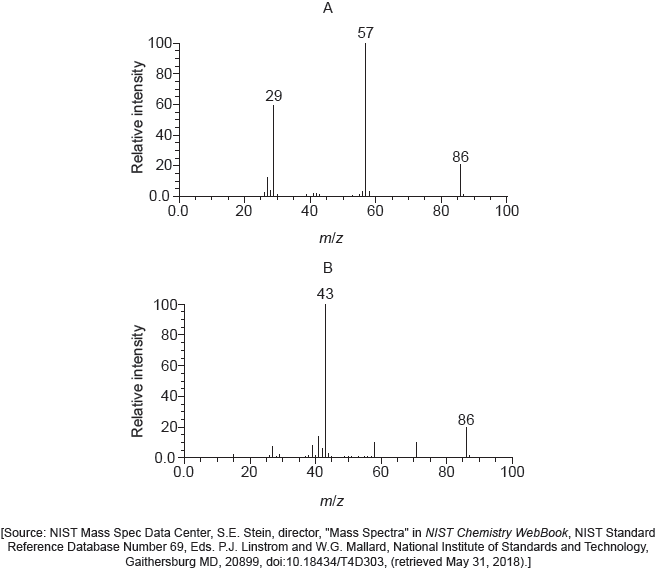
Explain which spectrum is produced by each compound using section 28 of the data booklet.
-
18M.2.hl.TZ2.9b.ii:
State the type of solvent most suitable for the reaction.
-
21N.1.hl.TZ0.16:
Consider the Born–Haber cycle for the formation of sodium oxide:
What is the lattice enthalpy, in kJ mol−1, of sodium oxide?
A. 414 + 2(108) + 249 + 2(496) − 141 + 790B. 414 + 2(108) + 249 + 2(496) + 141 + 790
C. −414 + 2(108) + 249 + 2(496) − 141 + 790
D. −414 − 2(108) − 249 − 2(496) + 141 − 790
- 21N.1.hl.TZ0.17: In which of the following situations is the forward reaction spontaneous? A. The equilibrium...
- 21N.2.hl.TZ0.10d: Deduce, with a reason, the mechanism of the reaction between 2-chloropentane and sodium hydroxide.
- 21N.2.hl.TZ0.10e: Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
- 18N.2.hl.TZ0.9d: State, giving your reason, whether the hydroxide ion acts as a Lewis acid, a Lewis base, or...
- 18N.2.hl.TZ0.10a: Classify substances B and D as reactant, product, catalyst, or intermediate, based on the...
- 18N.2.hl.TZ0.6a.ii: Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
-
22M.1.hl.TZ1.5:
The graph shows the first six ionization energies of an element.
[Ionization energies of the elements (data page) Available at: https://en.wikipedia.org/wiki/Ionization_energies_of_the_
elements_(data_page) Text is available under the Creative Commons Attribution-ShareAlike License 3.0 (CC BY-SA
3.0) https://creativecommons.org/licenses/by-sa/3.0/deed.en.]
In which group is the element?A. 13
B. 14
C. 15
D. 16
-
22M.1.hl.TZ1.8:
Why is hydrated copper (II) sulfate blue?
A. Blue light is emitted when electrons return to lower d-orbitals.
B. Light complimentary to blue is absorbed when electrons return to lower d-orbitals.
C. Blue light is emitted when electrons are promoted between d-orbitals.
D. Light complimentary to blue is absorbed when electrons are promoted between d-orbitals.
- 22M.1.hl.TZ2.17: Which term in the expression ΔG⦵ = ΔH⦵ − TΔS⦵ is an indirect measure of the entropy change of the...
- 22M.1.hl.TZ2.7: Which of these ions are likely to be paramagnetic? I. Ti3+II. Cr3+III. Fe3+ A. I and II...
-
22M.2.hl.TZ1.2b(ii):
Calculate the free energy change, ΔG⦵, in kJ, of the cell reaction. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.3a(ii):
Determine, giving a reason, if iodine will also oxidize iron(II).
-
22M.2.hl.TZ2.7b(i):
Sketch the shape of one sigma () and one pi () bond.
-
19M.2.hl.TZ1.2i:
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
-
19M.2.hl.TZ2.2c(i):
Deduce how the rate of reaction at t = 2 would compare to the initial rate.
-
19M.2.hl.TZ2.5f:
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
- 19M.1.hl.TZ1.13: How many carbon atoms are sp3, sp2 and sp hybridized in the molecule?
-
19M.1.hl.TZ2.37:
Which class of compound is formed when a ketone is reduced?
A. primary alcohol
B. secondary alcohol
C. ether
D. carboxylic acid
-
19N.2.hl.TZ0.1e(ii):
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
-
19N.2.hl.TZ0.6e(iii):
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
-
19N.2.hl.TZ0.4a(v):
Comment on the spontaneity of the reaction at 298 K.
-
19N.2.hl.TZ0.6d:
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
- 19N.1.hl.TZ0.21: Which is correct?
-
16N.1.hl.TZ0.28:
Which mixture is a buffer solution?
A. 25 cm3 of 0.10 mol dm-3 NH3 (aq) and 50 cm3 of 0.10 mol dm-3 HCl (aq)
B. 50 cm3 of 0.10 mol dm-3 NH3 (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
C. 25 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
D. 50 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
-
16N.1.hl.TZ0.40:
Which property explains why tetramethylsilane, Si(CH3)4, can be used as a reference standard in 1H NMR spectroscopy?
A. It has a high boiling point.
B. It is a reactive compound.
C. All its protons are in the same chemical environment.
D. It gives multiple signals.
- 16N.1.hl.TZ0.18: Which represents the enthalpy change of hydration of the chloride ion?
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 20N.1.hl.TZ0.30: Which conditions deposit the greatest mass of copper when solutions containing copper ions are...
-
20N.1.hl.TZ0.21:
Which graph represents the relationship between the rate constant, , and temperature, , in kelvin?
-
20N.2.hl.TZ0.6c:
The electron configuration of copper makes it a useful metal.
Copper plating can be used to improve the conductivity of an object.
State, giving your reason, at which electrode the object being electroplated should be placed.
-
20N.2.hl.TZ0.5b(iii):
Suggest, giving a reason, which point on the curve is considered a buffer region.
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
17M.1.hl.TZ1.5:
Which statement explains one of the decreases in first ionization energy (I.E.) across period 3?
A. The nuclear charge of element Al is greater than element Mg.
B. The electron-electron repulsion is greater, for the electron with the opposite spin, in element S than in element P.
C. A new sub-level is being filled at element S.
D. The p orbital being filled in element Al is at a lower energy than the s orbital in element Mg.
-
17M.1.hl.TZ1.30:
Which statement is correct for the overall reaction in a voltaic cell?
2AgNO3(aq) + Ni(s) → 2Ag(s) + Ni(NO3)2(aq) E θ= +1.06 V
A. Electrons flow from Ag electrode to Ni electrode.
B. Ni is oxidized to Ni2+ at the cathode (negative electrode).
C. Ag+ is reduced to Ag at the anode (positive electrode).
D. Ag has a more positive standard electrode potential value than Ni.
-
17M.2.hl.TZ1.4d:
Suggest why experiments involving tetracarbonylnickel are very hazardous.
-
17M.2.hl.TZ1.5b:
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
-
17M.2.hl.TZ1.8a:
Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
- 17M.1.hl.TZ2.13: Which statement is correct? A. Sigma bonds are formed only by the combination of s atomic...
- 17M.1.hl.TZ2.17: Which ion’s hydration energy is the most exothermic? A. Li+ B. Na+ C. Br– D. I–
- 17M.1.hl.TZ2.40: Which technique can be used to identify bond length and bond angle? A. 1H NMR...
-
17M.2.hl.TZ2.5b.v:
Sketch the relationship between the rate of reaction and the concentration of NO2.
-
17M.2.hl.TZ2.7c.i:
State the reagents and the name of the mechanism for the nitration of benzene.
-
17M.2.hl.TZ2.7d:
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
- 17N.1.hl.TZ0.39: Which compound gives this 1H NMR spectrum? A. CH3CH2OCH2CH3 B. CH3CH2OH C. CH3CH2CH3 D....
- 17N.2.hl.TZ0.8a.iv: Deduce, giving a reason, which of the two compounds can show optical activity.
-
17N.2.hl.TZ0.2e.ii:
State the rate expression for the reaction.
- 17N.1.hl.TZ0.35: What is the product of the reaction between pentan-2-one and sodium borohydride, NaBH4? A....
- 17N.1.hl.TZ0.15: Which statements are correct for ionic compounds? I. Lattice energy increases as ionic radii...
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
- 21M.1.hl.TZ1.12: Which contain delocalised electrons? I. C6H5OHII. CH3COO−III. CO32− A. I and II only B. I...
- 21M.1.hl.TZ2.13: What is the electron domain geometry of Si in SiO2? A. bent B. linear C. square planar D. ...
- 21M.2.hl.TZ1.1d(ii): State a technique that could be used to determine the crystal structure of the solid compound.
- 21M.2.hl.TZ1.1c: Sketch the first eight successive ionisation energies of sulfur.
-
21M.2.hl.TZ1.5e(i):
Sketch the mechanism for the reaction of propene with hydrogen bromide using curly arrows.
- 21M.2.hl.TZ1.4e(iv): Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the...
-
21M.2.hl.TZ1.8a:
Calculate the pH of 0.00100 mol dm–3 propanoic acid solution. Use section 21 of the data booklet.
-
21M.2.hl.TZ2.2b(iii):
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
-
21M.2.hl.TZ2.3d:
Calculate the Gibbs free energy change, ΔG⦵, in kJ, for the cell, using section 1 of the data booklet.
-
21M.2.hl.TZ2.4d:
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
- 18M.1.hl.TZ1.13: Which can be represented with only one Lewis structure? A. CH2O B. C6H6 C. O3 D. ...
- 18M.1.hl.TZ2.21: Which statement is correct? A. The value of the rate constant, k, is independent of...
-
18M.2.hl.TZ2.5e:
Determine the temperature, in K, above which the reaction becomes spontaneous.
- 18M.1.hl.TZ1.19: What are correct labels for the Maxwell−Boltzmann energy distribution curves?
- 18M.1.hl.TZ2.26: Which is an example of a Lewis base? A. an electrophile B. BF3 C. CH4 D. a...
- 18M.1.hl.TZ1.8: Which complex has the greatest d orbital splitting?
- 18M.1.hl.TZ2.12: Which molecule has an expanded octet? A. CO B. CO2 C. SF2 D. SF4
-
18M.1.hl.TZ2.17:
Which system has the most negative entropy change, ΔS, for the forward reaction?
A. N2(g) + 3H2(g) 2NH3(g)
B. CaCO3(s) → CaO(s) + CO2(g)
C. 2S2O32−(aq) + I2(aq) → S4O62−(aq) + 2I–(aq)
D. H2O(l) → H2O(g)
-
18M.2.hl.TZ1.7a:
Compare and contrast the mechanisms by which 1-chlorobutane, CH3CH2CH2CH2Cl, and 2-chloro-2-methylpropane, (CH3)3CCl, react with aqueous sodium hydroxide, giving two similarities and one difference.
-
18M.2.hl.TZ2.4c:
Calculate the standard electrode potential, in V, for the BrO3−/Br− reduction half‑equation using section 24 of the data booklet.
-
18M.2.hl.TZ2.7c.i:
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.
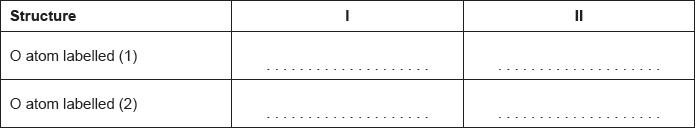
- 18M.1.hl.TZ1.29: What are the products of electrolysis when concentrated calcium bromide solution is electrolysed...
-
21N.1.hl.TZ0.30:
Consider the following standard electrode potentials:
Which species will react with each other spontaneously under standard conditions?
A. Zn2+ (aq) + Pb (s)B. Pb2+ (aq) + Br2 (l)
C. Zn (s) + Br− (aq)
D. Pb (s) + Br2 (l)
- 21N.1.hl.TZ0.35: Which statement is correct about configurational isomers? A. Configurational isomers can only...
-
21N.1.hl.TZ0.31:
Which aqueous solutions produce oxygen gas during electrolysis?
I. Dilute CuCl2 (aq) with inert electrodes
II. Dilute FeSO4 (aq) with inert electrodes
III. Dilute CuCl2 (aq) with copper electrodesThe standard electrode potentials are provided in the table:
A. I and II onlyB. I and III only
C. II and III only
D. I, II and III
- 21N.2.hl.TZ0.10b(iii): Explain the mechanism of the reaction between but-1-ene with hydrogen iodide, using curly arrows...
-
21N.2.hl.TZ0.10c(iii):
Determine the initial rate of reaction in experiment 4.
- 18N.1.hl.TZ0.35: Which statement about the reaction of a hydroxide ion with the organic reagent is...
-
18N.1.hl.TZ0.17:
Which change is exothermic?
A. Cl2 (g) → Cl (g)
B. K (g) → K+ (g) + e−
C. KCl (s) → K+ (g) + Cl− (g)
D. Cl (g) + e− → Cl− (g)
-
18N.1.hl.TZ0.27:
An indicator, HIn, has a pKa of 5.1.
HIn (aq) H+ (aq) + In− (aq)
colour A colour B
Which statement is correct?
A. At pH = 7, colour B would be observed
B. At pH = 3, colour B would be observed
C. At pH = 7, [HIn] = [In−]
D. At pH = 3, [HIn] < [In−] -
18N.1.hl.TZ0.8:
Which is correct for the complex ion in [Fe(H2O)5Cl]SO4?
- 22M.1.hl.TZ2.12: What is the molecular geometry of SF4? A. Tetrahedral B. Trigonal bipyramidal C. ...
- 22M.1.hl.TZ2.15: What are the signs of ΔH and ΔS for a reaction that is non-spontaneous at low temperatures but...
-
22M.2.hl.TZ2.4a(iii):
Calculate the value of the rate constant stating its units.
-
22M.2.hl.TZ2.4a(i):
Deduce the order of reaction with respect to hydrogen.
-
22M.2.hl.TZ2.8d(i):
Draw the full structural formula of (Z)-but-2-ene.
- 22M.2.hl.TZ2.5a(ii): Explain why there is a large increase from the 8th to the 9th ionization energy of iron.
-
22M.2.hl.TZ2.8d(v):
Predict, giving a reason, the major product of reaction between but-1-ene and steam.
- 22M.2.hl.TZ2.7d: Explain why transition metal cyanide complexes are coloured.
-
19M.2.hl.TZ1.5d(i):
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
-
19M.2.hl.TZ1.5c(ii):
State the hybridization of the nitrogen atom in chloramine.
-
19M.2.hl.TZ1.6f(ii):
Calculate ΔGθ, in kJ, for the spontaneous reaction in (f)(i), using sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ2.2g(ii):
Dinitrogen monoxide has a positive standard enthalpy of formation, ΔHfθ.
Deduce, giving reasons, whether altering the temperature would change the spontaneity of the decomposition reaction.
-
19M.2.hl.TZ2.1c(v):
Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
-
19M.2.hl.TZ2.2g(i):
Determine the standard entropy change, in J K−1, for the decomposition of dinitrogen monoxide.
2N2O (g) → 2N2 (g) + O2 (g)
- 19M.1.hl.TZ1.5: Which element is represented by the first eight successive ionization energies on the...
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ1.36: Which statement is not correct regarding benzene? A. It is planar. B. The ring contains...
-
19M.1.hl.TZ2.16:
Which equation represents lattice enthalpy?
A. NaCl (g) → Na+ (g) + Cl− (g)
B. NaCl (s) → Na+ (g) + Cl− (g)
C. NaCl (s) → Na+ (aq) + Cl− (aq)
D. NaCl (s) → Na+ (s) + Cl− (s)
-
19M.1.hl.TZ2.17:
Which change has the greatest increase in entropy?
A. CO2 (s) → CO2 (g)
B. CO2 (g) → CO2 (l)
C. CO2 (g) → CO2 (s)
D. CO2 (l) → CO2 (s)
-
19M.1.hl.TZ2.12:
How many sigma (σ) and pi (π) bonds are present in hydrogen cyanide, HCN?
-
19N.2.hl.TZ0.6f(i):
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
-
19N.2.hl.TZ0.3b(iii):
Sketch the mechanism using curly arrows to represent the movement of electrons.
-
19N.2.hl.TZ0.6e(iv):
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
- 19N.1.hl.TZ0.12: Which atom is sp2 hybridized? A. C in H2CO B. C in CO2 C. N in CH3NH2 D. O in H2O
-
19N.1.hl.TZ0.17:
Which reaction has the greatest increase in entropy of the system?
A. HCl (g) + NH3 (g) → NH4Cl (s)
B. (NH4)2Cr2O7 (s) → Cr2O3 (s) + N2 (g) + 4H2O (g)
C. CaCO3 (s) → CaO (s) + CO2 (g)
D. I2 (g) → I2 (s)
- 19N.1.hl.TZ0.13: Which atom does not obey the octet rule? A. C in CO2 B. F in BF3 C. O in H2O D. S in SF6
- 16N.1.hl.TZ0.21: Which statement describes the characteristics of a transition state relative to the potential...
- 16N.1.hl.TZ0.6: A period 3 element, M, forms an oxide of the type M2O. Which represents the first four successive...
-
16N.1.hl.TZ0.29:
Which salt solution has the highest pH?
A. NH4Cl
B. Ca(NO3)2
C. Na2CO3
D. K2SO4
- 16N.1.hl.TZ0.15: What is the hybridization of the numbered atoms in ethanoic acid?
-
16N.2.hl.TZ0.5d:
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
-
20N.1.hl.TZ0.37:
Which molecule has an enantiomer?
A.
B.
C.
D.
-
20N.2.hl.TZ0.2c:
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
-
20N.2.hl.TZ0.1e(iii):
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
-
20N.2.hl.TZ0.1d(v):
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
- 20N.2.hl.TZ0.5b(ii): State a suitable indicator for this titration. Use section 22 of the data booklet
-
20N.2.hl.TZ0.4d(ii):
Calculate the standard cell potential, in , for the cell at . Use section 24 of the data booklet
-
20N.2.hl.TZ0.3e:
Calculate the standard Gibbs free energy change, , in , for the reaction at 5 °C, using your answers to (b) and (d). Use section 1 of the data booklet.
(If you did not obtain an answer to (b) or (d) use values of and respectively, although these are not the correct answers.)
-
20N.2.hl.TZ0.6a:
The electron configuration of copper makes it a useful metal.
Determine the frequency of a photon that will cause the first ionization of copper. Use sections 1, 2 and 8 of the data booklet.
-
20N.2.hl.TZ0.6b:
The electron configuration of copper makes it a useful metal.
Explain why a copper(II) solution is blue, using section 17 of the data booklet.
- 17M.1.hl.TZ1.37: Which molecule is chiral? A. 2-chlorobutane B. 2,2-dichloropentane C. ...
-
17M.1.hl.TZ1.40:
Which technique is used to determine the bond lengths and bond angles of a molecule?
A. X-ray crystallography
B. Infrared (IR) spectroscopy
C. Mass spectroscopy
D. 1H NMR spectroscopy
-
17M.2.hl.TZ1.6b.iii:
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
-
17M.1.hl.TZ2.8:
Ammonia is a stronger ligand than water. Which is correct when concentrated aqueous ammonia solution is added to dilute aqueous copper(II) sulfate solution?
A. The d-orbitals in the copper ion split.
B. There is a smaller splitting of the d-orbitals.
C. Ammonia replaces water as a ligand.
D. The colour of the solution fades.
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and respectively.
X + 2Y 2W + Z
What is the value of the equilibrium constant, Kc?
A.
B.
C. 2
D. 8
- 17M.1.hl.TZ2.30: What is the standard half-cell potential of copper if the “zero potential reference electrode”...
-
17M.2.hl.TZ2.4c:
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
-
17M.2.hl.TZ2.7c.ii:
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
-
17M.2.hl.TZ2.8b.iv:
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
-
17M.2.hl.TZ2.9b.i:
Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for the hydrogenation of propene.
-
17M.2.hl.TZ1.5c:
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium. -
21M.1.hl.TZ2.40:
What information can be deduced from the splitting pattern of 1H NMR signals?
A. total number of hydrogen atoms in a compound
B. number of hydrogen atoms on adjacent atom(s)
C. functional group on which hydrogen atoms are located
D. number of hydrogen atoms in a particular chemical environment
-
17N.2.hl.TZ0.7e:
State and explain the products of electrolysis of a concentrated aqueous solution of sodium chloride using inert electrodes. Your answer should include half-equations for the reaction at each electrode.
-
17N.1.hl.TZ0.21:
The rate expression for the reaction X (g) + 2Y (g) → 3Z (g) is
rate = k[X]0 [Y]2
By which factor will the rate of reaction increase when the concentrations of X and Y are both increased by a factor of 3?
A. 6
B. 9
C. 18
D. 27
-
17N.1.hl.TZ0.16:
What is the standard enthalpy of formation, in kJ mol–1, of IF (g)?
IF7 (g) + I2 (s) → IF5 (g) + 2IF (g) ΔH = –89 kJ
ΔH (IF7) = –941 kJ mol–1
ΔH (IF5) = –840 kJ mol–1
A. –190
B. –95
C. +6
D. +95
- 17N.2.hl.TZ0.3e: Describe, in terms of acid-base theories, the type of reaction that takes place between the...
-
17N.2.hl.TZ0.6c.i:
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
-
21M.1.hl.TZ1.17:
Which substance has the highest lattice enthalpy?
A.
B.
C.
D.
- 21M.1.hl.TZ1.6: The diagram shows the first ionisation energies of consecutive elements in the same period of the...
- 21M.1.hl.TZ1.27: Which combination will produce an alkaline buffer in water? A. 0.10 mol NH3 and 0.05 mol...
-
21M.1.hl.TZ1.31:
What are the products when concentrated aqueous copper (II) chloride is electrolysed using platinum electrodes?
- 21M.1.hl.TZ1.36: What is the product of the reaction of benzene with a mixture of concentrated nitric and sulfuric...
- 21M.1.hl.TZ2.20: Which graph represents a second order reaction with respect to X? X → Y
-
21M.2.hl.TZ1.4e(ii):
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
-
21M.2.hl.TZ1.6b(iv):
Calculate the value of the rate constant, k, giving its units.
-
21M.2.hl.TZ1.6b(iii):
Write the rate expression for this reaction.
-
21M.2.hl.TZ2.1b(iii):
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
- 18M.1.hl.TZ2.8: Part of the spectrochemical series is shown for transition metal complexes. I−< Cl− < H2O...
- 18M.1.hl.TZ1.26: Which statements are correct? I. Lewis bases can act as nucleophiles. II....
- 18M.1.hl.TZ2.20: When X reacts with Y to give Z, the following graph is plotted. What can be deduced from the...
- 18M.1.hl.TZ2.27: What is the order of increasing acidity? A. HClO < CH3CH2COOH < HF < HIO3 B. ...
-
18M.1.hl.TZ2.31:
What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?
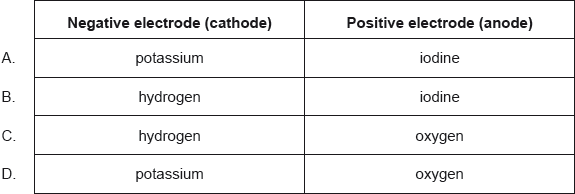
-
18M.2.hl.TZ1.4b.v:
Calculate the rate constant of the reaction, stating its units.
-
18M.2.hl.TZ1.5d:
Determine the pH of 0.010 mol dm−3 2,2-dimethylpropanoic acid solution.
Ka (2,2-dimethylpropanoic acid) = 9.333 × 10−6
-
18M.2.hl.TZ1.6d:
Calculate the cell potential, in V, using section 24 of the data booklet.
-
18M.2.hl.TZ1.6e:
Determine the loss in mass of one electrode if the mass of the other electrode increases by 0.10 g.
-
18M.2.hl.TZ2.5c:
The table lists standard entropy, SΘ, values.
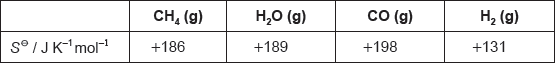
Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
-
21N.1.hl.TZ0.36:
Which product is formed when CH3COCH2CH3 is reduced with sodium borohydride?
A. CH3CH2CH2CHOB. CH3CH2CH2CH2OH
C. CH3CH(OH)CH2CH3
D. CH3CH2CH2COOH
-
21N.2.hl.TZ0.9b(iii):
Copper is a transition metal that forms different coloured complexes. A complex [Cu(H2O)6]2+ (aq) changes colour when excess Cl− (aq) is added.
Explain the cause of this colour change, using sections 3 and 15 from the data booklet.
-
21N.2.hl.TZ0.11c:
Sketch the neutralisation curve obtained and label the equivalence point.
-
21N.2.hl.TZ0.10a(i):
Distinguish between a sigma and pi bond.
-
18N.1.hl.TZ0.31:
Consider the standard electrode potentials:
Cr3+ (aq) + 3e− Cr (s) EΘ = −0.74 V
Hg2+ (aq) + 2e− Hg (l) EΘ = +0.85 V
What is the cell potential, in V, for the voltaic cell?
2Cr (s) + 3Hg2+ (aq) → 3Hg (l) + 2Cr3+ (aq)
A. −1.59
B. +0.11
C. +1.07
D. +1.59
- 18N.1.hl.TZ0.13: What is the hybridization of the circled carbon, oxygen and nitrogen atoms?
-
18N.2.hl.TZ0.3e:
State and explain the magnetic property of iron(II) and iron(III) ions.
-
18N.2.hl.TZ0.5d:
Predict, giving your reasons, whether the forward reaction is endothermic or exothermic. Use your answers to (b) and (c).
-
18N.2.hl.TZ0.3b.i:
Draw two Lewis (electron dot) structures for BrO3−.
- 18N.2.hl.TZ0.4d: Predict, giving your reasons, whether Mn2+ or Fe2+ is likely to have a more exothermic enthalpy...
-
18N.3.hl.TZ0.1c:
Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
-
22M.1.hl.TZ1.17:
In which reaction does entropy decrease?
A. NaCl (s) → NaCl (aq)
B. Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
C. NH3 (g) + HCl (g) → NH4Cl (s)
D. CuCO3 (s) → CuO (s) + CO2 (g)
- 22M.1.hl.TZ1.37: What are the E/Z designations of these stereoisomers?
- 22M.1.hl.TZ1.20: The table shows data for the hydrolysis of a halogenoalkane, RCl. Which statements are...
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.2c(i):
Use the graph to deduce the dependence of the reaction rate on the amount of Mg.
-
22M.2.hl.TZ1.4d:
Magnesium salts form slightly acidic solutions owing to equilibria such as:
Mg2+ (aq) + H2O (l) Mg(OH)+ (aq) + H+ (aq)
Comment on the role of Mg2+ in forming the Mg(OH)+ ion, in acid-base terms.
-
22M.2.hl.TZ1.6b(iii):
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
-
22M.2.hl.TZ1.6a(ii):
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
-
22M.2.hl.TZ1.6a(iii):
Explain the relative lengths of the three bonds between N and O in nitric acid.
-
22M.2.hl.TZ2.4a(ii):
Deduce the rate expression for the reaction.
-
22M.2.hl.TZ2.4d(ii):
Predict, giving a reason, how the value of the ΔS⦵reaction would be affected if (g) were used as a reactant.
- 22M.2.hl.TZ2.7a(i): State why NH3 is a Lewis base.
-
19M.2.hl.TZ1.6b:
Explain why, when ligands bond to the iron ion causing the d-orbitals to split, the complex is coloured.
-
19M.2.hl.TZ2.3a(ii):
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
-
19M.2.hl.TZ2.3d(iii):
Deduce the hybridization of the central nitrogen atom in the molecule.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
- 19M.1.hl.TZ1.37: Which compound can exist as cis- and trans-isomers?
-
19M.1.hl.TZ2.21:
What is the order with respect to each reactant?
2NO (g) + Cl2 (g) → 2NOCl (g)
-
19M.1.hl.TZ1.8:
Which electrons are removed from iron (Z = 26) to form iron(II)?
A. two 3d electrons
B. two 4s electrons
C. one 4s electron and one 3d electron
D. two 4p electrons
-
19M.1.hl.TZ1.33:
Which is a major product of the electrophilic addition of hydrogen chloride to propene?
A. ClCH2CH=CH2
B. CH3CH(Cl)CH3
C. CH3CH2CH2Cl
D. CH3CH=CHCl
-
19M.1.hl.TZ2.8:
What is the oxidation state of the metal ion and charge of the complex ion in [Co(NH3)4Cl2]Cl?
-
19M.1.hl.TZ2.31:
What are the products when concentrated KBr (aq) is electrolyzed?
-
19N.2.hl.TZ0.3a(iv):
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
-
19N.1.hl.TZ0.18:
What is the order of increasing (more exothermic) enthalpy of hydration?
Xn+ (g) → Xn+ (aq)
A. Ca2+, Mg2+, K+, Na+
B. Na+, K+, Mg2+, Ca2+
C. K+, Na+, Ca2+, Mg2+
D. Mg2+, Ca2+, Na+, K+
-
19N.2.hl.TZ0.6f(iv):
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
-
19N.2.hl.TZ0.4a(iv):
Calculate the standard Gibbs free energy change, , in kJ mol−1, for the first dissociation of citric acid at 298 K, using section 1 of the data booklet.
-
19N.2.hl.TZ0.6f(iii):
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
- 19N.2.hl.TZ0.5a: A sample of ethanoic acid was titrated with sodium hydroxide solution, and the following pH curve...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
-
16N.2.hl.TZ0.1b:
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
-
16N.2.hl.TZ0.4i:
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
-
16N.1.hl.TZ0.9:
The oxidation state of cobalt in the complex ion [Co(NH3)5Br]x is +3. Which of the following statements are correct?
I. The overall charge, x, of the complex ion is 2+.
II. The complex ion is octahedral.
III. The cobalt(III) ion has a half-filled d-subshell.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 16N.1.hl.TZ0.19: Which ionic compound has the largest value of lattice enthalpy? A. MgS B. MgO C. CaBr2 D. NaF
- 16N.2.hl.TZ0.6a: Draw the three-dimensional shape of each enantiomer of this isomer showing their spatial...
- 20N.1.hl.TZ0.5: Which element is in group 13?
-
20N.1.hl.TZ0.16:
Which combination gives the standard hydration enthalpy of ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.20:
What are the units of the rate constant, , if the rate equation is ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.35:
Which is the electrophile in the nitration of benzene?
A.
B.
C.
D.
-
20N.2.hl.TZ0.2b:
State the type of hybridization shown by the central carbon atom in molecule B.
-
20N.2.hl.TZ0.5b(i):
Identify the major species, other than water and potassium ions, at these points.
-
20N.2.hl.TZ0.3c:
Predict, giving a reason, whether the entropy change, , for this reaction is negative or positive.
-
20N.3.hl.TZ0.13b:
Calculate the cell potential for and at . Use sections 1, 2 and 24 of the data booklet.
- 17M.1.hl.TZ1.17: Which combination of ΔH θ and ΔS θ will result in a non-spontaneous reaction at all temperatures?
-
17M.1.hl.TZ1.21:
What are the units for the rate constant, k, in the expression?
Rate = k [X]2[Y]
A. mol2 dm−6 s−1
B. mol−1 dm3 s−1
C. mol dm−3 s−1
D. mol−2 dm6 s−1
-
17M.2.hl.TZ1.1d:
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
-
17M.2.hl.TZ1.2d.iii:
Sketch a graph of the first six successive ionization energies of vanadium on the axes provided.
-
17M.2.hl.TZ1.2e:
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
-
17M.2.hl.TZ1.6b.ii:
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
-
17M.2.hl.TZ1.8b:
Suggest why the loss of ozone is an international environmental concern.
- 17M.1.hl.TZ2.10: Which does not show resonance? A. PO43– B. C6H6 C. C6H12 D. O3
- 17M.1.hl.TZ2.12: Which is the first step in the CFC-catalysed destruction of ozone in UV light? A. CCl2F2 →...
-
17M.1.hl.TZ2.37:
In which order should the reagents be used to convert benzene into phenylamine (aniline)?
-
17M.2.hl.TZ2.8b.vi:
Deduce the pKa for this acid.
- 17N.2.hl.TZ0.2e.iii: Calculate the value of the rate constant at 263 K.
-
17N.1.hl.TZ0.14:
How many sigma (σ) and pi (π) bonds are present in this molecule?
- 17N.1.hl.TZ0.26: Which of the following will form a buffer solution if combined in appropriate molar ratios? A....
- 17N.2.hl.TZ0.3d.i: State the shape of the complex ion.
-
17N.2.hl.TZ0.6c.ii:
Calculate Kb for HCO3– acting as a base.
- 17N.1.hl.TZ0.22: Which pair of statements explains the increase in rate of reaction when the temperature...
-
17N.2.hl.TZ0.8d:
State an equation for the formation of NO2+.
-
21M.1.hl.TZ1.9:
What is the overall charge, , of the chromium (III) complex?
A. 0
B. 1+
C. 2−
D. 3+
-
21M.1.hl.TZ1.20:
A reaction proceeds by the following mechanism:
step 1:
step 2:Which rate equation is consistent with this mechanism?
A. Rate = k[B]2[C]
B. Rate = k[A]2[B][C]
C. Rate = k[A]2
D. Rate = k[A][C]
- 21M.1.hl.TZ1.37: How many chiral centres are there in the following molecule? A. 2 B. 3 C. 4 D. 6
- 21M.2.hl.TZ1.3f: Outline why, unlike typical transition metals, zinc compounds are not coloured.
-
21M.2.hl.TZ2.2b(iv):
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
-
21M.2.hl.TZ2.6a:
Determine the rate expression for the reaction.
-
21M.2.hl.TZ2.5d(ii):
Identify an indicator that could be used for the titration in 5(d)(i), using section 22 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
- 18M.1.hl.TZ2.37: Which isomers exist as non-superimposable mirror images? A. cis-trans isomers B. ...
-
18M.2.hl.TZ1.1l.ii:
Predict the splitting pattern of the 1H NMR spectrum of urea.
-
18M.2.hl.TZ2.2d.ii:
Calculate the pH of 0.100 mol dm−3 aqueous ethanoic acid.
Ka = 1.74 × 10−5
- 18M.1.hl.TZ2.5: The graph shows the first ionization energies of some consecutive elements. Which statement is...
- 18M.1.hl.TZ2.35: Which is the correct combination of substitution reaction mechanisms?
-
18M.2.hl.TZ1.2e:
Sketch a graph of the first six ionization energies of calcium.
-
18M.2.hl.TZ1.4d:
Describe how the activation energy of this reaction could be determined.
-
18M.2.hl.TZ2.2d.iv:
Predict whether the pH of an aqueous solution of ammonium chloride will be greater than, equal to or less than 7 at 298 K.
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
-
18M.2.hl.TZ2.3d:
Explain why transition metals exhibit variable oxidation states in contrast to alkali metals.
-
18M.2.hl.TZ2.9b.iv:
Suggest, giving a reason, the percentage of each isomer from the SN1 reaction.
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
-
21N.1.hl.TZ0.12:
Which molecules contain two pi () bonds?
I. HCN
II. H2CO3
III. H2C2O4
A. I and II onlyB. I and III only
C. II and III only
D. I, II and III
- 21N.1.hl.TZ0.13: What is the hybridization of nitrogen and chlorine in NCl3?
-
21N.1.hl.TZ0.8:
Which complex ion contains a central ion with an oxidation state of +3?
A. [PtCl6]2−B. [Cu(H2O)4(OH)2]
C. [Ni(NH3)4(H2O)2]2+
D. [Co(NH3)4Cl2]+
- 21N.2.hl.TZ0.3b(ii): Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
- 21N.2.hl.TZ0.10c(ii): Deduce the units of the rate constant.
-
21N.2.hl.TZ0.3c(ii):
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
-
21N.2.hl.TZ0.11b:
The concentration of excess sodium hydroxide was 0.362 mol dm−3. Calculate the pH of the solution at the end of the experiment.
- 21N.2.hl.TZ0.10b(i): State, giving a reason, if but-1-ene exhibits cis-trans isomerism.
-
21N.2.hl.TZ0.11a:
Calculate the initial pH before any sodium hydroxide was added, using section 21 of the data booklet.
- 18N.1.hl.TZ0.26: Which species is not a Lewis base? A. OH− B. NH4+ C. H2O D. PH3
- 18N.2.hl.TZ0.10c: Calculate the initial rate of reaction for experiment 2, if measured under the same conditions.
-
18N.2.hl.TZ0.1d:
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
- 18N.2.hl.TZ0.8b.ii: State, giving a reason, whether methyloxirane can form cis-trans isomers.
-
18N.2.hl.TZ0.3d.iii:
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
- 22M.1.hl.TZ1.16: Which compound has the largest value of lattice enthalpy? A. Na2O B. K2O C. Na2S D. K2S
-
22M.1.hl.TZ2.14:
Which equation represents hydration enthalpy?
A. Na+ (g) → Na+ (aq)
B. Na+ (aq) → Na+ (g)
C. NaCl (s) → NaCl (aq)
D. NaCl (aq) → NaCl (s)
- 22M.2.hl.TZ1.1d(ii): Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that...
- 22M.2.hl.TZ1.6b(ii): Draw the structural formula of the carbocation intermediate produced when this electrophile...
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
-
19M.2.hl.TZ1.3d(iii):
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
-
19M.2.hl.TZ1.1c(ii):
Explain the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
-
19M.2.hl.TZ2.4b(ii):
A scientist wants to investigate the catalytic properties of a thin layer of rhenium metal on a graphite surface.
Describe an electrochemical process to produce a layer of rhenium on graphite.
-
19M.2.hl.TZ2.4b(iii):
Predict two other chemical properties you would expect rhenium to have, given its position in the periodic table.
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
-
19M.1.hl.TZ1.16:
Which is correct for the reaction H2O (g) → H2O (l) ?
A. Enthalpy increases and entropy increases.
B. Enthalpy decreases and entropy increases.
C. Enthalpy increases and entropy decreases.
D. Enthalpy decreases and entropy decreases.
-
19M.1.hl.TZ2.5:
Which of the following transitions in the hydrogen atom emits the least energy?
A. n = 2 to n = 1
B. n = 3 to n = 1
C. n = 4 to n = 2
D. n = 4 to n = 3
- 19M.1.hl.TZ2.13: What is the hybridization of carbon and oxygen in methanol?
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
-
19N.2.hl.TZ0.6c(iii):
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
- 19N.1.hl.TZ0.37: Which can be reduced to an aldehyde? A. Butanone B. Butan-1-ol C. Butanoic acid D. Butan-2-ol
- 19N.1.hl.TZ0.27: Which can act as a Lewis acid but not a Brønsted–Lowry acid? A. BF3 B. H2O C. NF3 D. NH3
-
19N.2.hl.TZ0.5b(ii):
Describe, using a suitable equation, how the buffer solution formed during the titration resists pH changes when a small amount of acid is added.
- 19N.2.hl.TZ0.6e(i): Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24...
- 16N.1.hl.TZ0.37: Which statement is correct for a pair of enantiomers under the same conditions? A. A racemic...
- 16N.2.hl.TZ0.2f: Explain how ethanedioate ions act as ligands.
-
16N.1.hl.TZ0.10:
What is the correct explanation for the colour of [Cu(H2O)6]2+?
A. Light is absorbed when an electron moves to a d orbital of higher energy.
B. Light is released when an electron moves to a d orbital of higher energy.
C. Light is absorbed when electrons move from the ligands to the central metal ion.
D. Light is absorbed when electrons move between d and s orbitals.
-
16N.2.hl.TZ0.3e:
(i) Using the graph, explain the order of reaction with respect to sodium thiosulfate.
(ii) In a different experiment, this reaction was found to be first order with respect to hydrochloric acid. Deduce the overall rate expression for the reaction.
-
16N.1.hl.TZ0.33:
An iron rod is electroplated with silver. Which is a correct condition for this process?
A. The silver electrode is the positive electrode.
B. The iron rod is the positive electrode.
C. The electrolyte is iron(II) sulfate.
D. Oxidation occurs at the negative electrode.
-
20N.1.hl.TZ0.11:
Which combination correctly describes the geometry of ?
- 17M.1.hl.TZ1.13: Which species have resonance structures? I. Ozone, O3II. Carbon dioxide, CO2III. ...
-
17M.2.hl.TZ1.2f:
Outline why transition metals form coloured compounds.
-
17M.2.hl.TZ1.5d.ii:
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
-
17M.2.hl.TZ1.7e:
State the reagents used in the two-stage conversion of nitrobenzene to aniline.
-
17M.1.hl.TZ2.20:
Which is true of an Arrhenius plot of (y-axis) against ?
A. The graph goes through the origin.
B. The activation energy can be determined from the gradient.
C. The intercept on the x-axis is the activation energy.
D. The intercept on the y-axis is the frequency factor, A.
- 17M.1.hl.TZ2.21: Which is correct about reaction mechanisms? A. A species that is zero order does not take...
- 17M.1.hl.TZ2.27: What is the order of increasing acidity of the following acids? A. chloroethanoic <...
-
17M.2.hl.TZ2.2b.i:
Corrosion of iron is similar to the processes that occur in a voltaic cell. The initial steps involve the following half-equations:
Fe2+(aq) + 2e– Fe(s)
O2(g) + H2O(l) + 2e– 2OH–(aq)
Calculate E θ, in V, for the spontaneous reaction using section 24 of the data booklet.
-
17M.2.hl.TZ2.2b.iii:
Explain why iron forms many different coloured complex ions.
-
17M.2.hl.TZ2.2c:
Zinc is used to galvanize iron pipes, forming a protective coating. Outline how this process prevents corrosion of the iron pipes.
-
17M.2.hl.TZ2.5b.iii:
State one method that can be used to measure the rate for this reaction.
-
17M.2.hl.TZ2.5c:
The Arrhenius equation, , gives the relationship between the rate constant and temperature.
State how temperature affects activation energy.
-
17M.2.hl.TZ2.8c:
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
-
17M.2.hl.TZ1.2d.iv:
Explain why an aluminium-titanium alloy is harder than pure aluminium.
-
21M.2.hl.TZ1.5e(ii):
Explain why the major organic product is 2-bromopropane and not 1-bromopropane.
- 17N.1.hl.TZ0.37: What is the number of optical isomers of isoleucine? A. 0 B. 2 C. 4 D. 8
- 17N.2.hl.TZ0.2e.i: Deduce the order of reaction with respect to Cl2 and NO.
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
-
17N.1.hl.TZ0.10:
[CoCl6]3– is orange while [Co(NH3)6]3+ is yellow. Which statement is correct?
A. [CoCl6]3– absorbs orange light.
B. The oxidation state of cobalt is different in each complex.
C. The different colours are due to the different charges on the complex.
D. The different ligands cause different splitting in the 3d orbitals.
-
17N.1.hl.TZ0.27:
Which indicator is appropriate for the acid-base titration shown below?
A. Thymol blue (pKa = 1.5)
B. Methyl orange (pKa = 3.7)
C. Bromophenol blue (pKa = 4.2)
D. Phenolphthalein (pKa = 9.6) -
17N.2.hl.TZ0.5c:
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
-
17N.2.hl.TZ0.7b:
Predict, giving a reason, the direction of movement of electrons when the standard nickel and manganese half-cells are connected.
- 21M.1.hl.TZ1.13: In which series are all carbon atoms sp2 hybridized? A. C2H2 H2CO HCOOH B. C2H4 ...
- 21M.1.hl.TZ1.30: Which gives the equation and cell potential of the spontaneous reaction?
-
21M.1.hl.TZ1.35:
Which is most likely to hydrolyse via a SN1 mechanism?
A. CH3CHBrCH2CH3
B. (CH3)2CHBr
C. (CH3)3CBr
D. CH3CH2CH2CH2Br
- 21M.1.hl.TZ2.8: Which factor does not affect the colour of a complex ion? A. temperature of the solution B. ...
-
21M.1.hl.TZ2.16:
Which represents electron affinity?
A. Al2+ (g) → Al3+ (g) + e−
B. C (g) + e− → C− (g)
C. Cl2 (g) → 2Cl (g)
D. S (s) → S+ (g) + e−
-
21M.1.hl.TZ2.36:
Which compound rotates the plane of plane-polarized light?
A. CH3C(CH3)ClCH3
B. CH3CH2CHClCH3
C. CH3C(Cl)2CH3
D. CH3CClBrCH3
- 21M.1.hl.TZ2.27: Which compound is acidic in aqueous solution? A. KBr B. CH3COONa C. NH4Cl D. Na2CO3
-
21M.1.hl.TZ2.31:
What happens to the mass of each copper electrode when aqueous copper(II) sulfate solution is electrolysed?
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
- 21M.2.hl.TZ1.4e(iii): Justify the sign of ΔS with reference to the equation.
- 21M.2.hl.TZ1.5b(ii): Deduce the chemical shift of this signal. Use section 27 of the data booklet.
- 21M.2.hl.TZ2.4e: Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly...
- 21M.2.hl.TZ2.4f: Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
-
21M.2.hl.TZ2.5a(ii):
Deduce the change in enthalpy, ΔH, in kJ, when 56.00 g of ethanol is burned. Use section 13 in the data booklet.
-
18M.1.hl.TZ1.16:
What is the enthalpy of solution of MgF2(s) in kJ mol−1?
Lattice enthalpy of MgF2(s) = 2926 kJ mol−1
Hydration enthalpy of Mg2+(g) = −1963 kJ mol−1
Hydration enthalpy of F−(g) = −504 kJ mol−1
A. 2926 − 1963 + 2(−504)
B. 2926 − 1963 − 504
C. −2926 − (−1963) − (−504)
D. −2926 − (−1963) − 2(−504)
- 18M.1.hl.TZ1.21: What is the effect of increasing temperature on the rate constant, k? A. The rate constant...
- 18M.1.hl.TZ1.31: What does not affect the mass of products formed in electrolysis of an aqueous solution? A. ...
- 18M.1.hl.TZ1.35: What is name of this compound applying IUPAC rules? A. E 1-bromo-1-chlorobut-1-ene B. ...
-
18M.2.hl.TZ1.1i:
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
-
18M.2.hl.TZ1.3c.v:
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
-
18M.2.hl.TZ1.4b.iv:
Deduce the rate expression for the reaction.
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
-
18M.2.hl.TZ2.4b:
The change in the free energy for the reaction under standard conditions, ΔGΘ, is −514 kJ at 298 K.
Determine the value of EΘ, in V, for the reaction using sections 1 and 2 of the data booklet.
-
18M.2.hl.TZ2.9b.i:
State the type of bond fission that takes place in a SN1 reaction.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
-
21N.2.hl.TZ0.10c(i):
Deduce the rate expression for this reaction.
- 21N.2.hl.TZ0.10a(ii): Identify the hybridization of carbon in ethane, ethene and ethyne.
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.1.hl.TZ0.16:
What are the signs of ΔHΘ and ΔSΘ for the reaction, which is spontaneous at low temperature and non-spontaneous at very high temperature?
ΔGΘ = ΔHΘ − TΔSΘ
SO3 (g) + CaO (s) → CaSO4 (s)
-
18N.2.hl.TZ0.10b:
Deduce the rate expression.
-
22M.1.hl.TZ1.31:
In the electrolysis apparatus shown, 0.59 g of Ni is deposited on the cathode of the first cell.
What is the mass of Ag deposited on the cathode of the second cell?
A. 0.54 gB. 0.59 g
C. 1.08 g
D. 2.16 g
-
22M.1.hl.TZ1.30:
What are the products when dilute aqueous copper (II) nitrate is electrolysed using platinum electrodes?
E⦵ (Cu | Cu2+) = –0.34 V.
- 22M.1.hl.TZ2.20: Which energy profile diagram represents an exothermic SN1 reaction?
- 22M.2.hl.TZ1.1e(iv): Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization...
-
22M.2.hl.TZ1.2b(iii):
This cell causes the electrolytic reduction of water on the steel. State the half-equation for this reduction.
-
22M.2.hl.TZ1.5a(iii):
State the number of (sigma) and (pi) bonds in Compound A.
-
22M.2.hl.TZ1.5a(v):
Identify the isomer of Compound B that exists as optical isomers (enantiomers).
-
22M.2.hl.TZ1.5b(ii):
Explain why the reaction produces more (CH3)3COH than (CH3)2CHCH2OH.
-
22M.2.hl.TZ1.6b(i):
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
-
22M.2.hl.TZ2.4d(i):
Calculate the entropy change of reaction, ΔS⦵, in J K−1 mol−1.
-
19M.2.hl.TZ1.2a:
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
-
19M.2.hl.TZ1.3a:
Plot the relative values of the first four ionization energies of sodium.
-
19M.2.hl.TZ1.3d(i):
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1O2(g) → O2- (g):
Na (s) → Na+ (g):
-
19M.2.hl.TZ1.2b:
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ1.6f(i):
Calculate the standard electrode potential, in V, when the Fe2+ (aq) | Fe (s) and Cu2+ (aq) | Cu (s) standard half-cells are connected at 298 K. Use section 24 of the data booklet.
-
19M.2.hl.TZ2.6d(ii):
The minor product, C6H5–CH2–CH2Br, can exist in different conformational forms (isomers).
Outline what this means.
-
19M.2.hl.TZ2.3c:
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
-
19M.2.hl.TZ2.6c:
The benzene ring of phenylethene reacts with the nitronium ion, NO2+, and the C=C double bond reacts with hydrogen bromide, HBr.
Compare and contrast these two reactions in terms of their reaction mechanisms.
Similarity:
Difference:
-
19M.1.hl.TZ2.20:
Which statement is correct about a catalyst?
A. It decreases the activation energy of the forward reaction but not the reverse.
B. It increases the proportion of products to reactants in an equilibrium.
C. It decreases the enthalpy change of the reaction.
D. It changes the mechanism of the reaction.
-
19M.1.hl.TZ1.40:
Which can be identified using infrared (IR) spectroscopy?
A. functional groups
B. molar mass
C. 3-D configuration
D. bond angle
- 19N.1.hl.TZ0.8: What is the effect of a stronger ligand?
- 19N.2.hl.TZ0.3b(ii): Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium...
-
19N.2.hl.TZ0.6f(ii):
Deduce why the Cu(I) solution is colourless.
- 19N.1.hl.TZ0.28: What is the order, in increasing pH, of the following solutions of equal concentration? A....
-
19N.1.hl.TZ0.32:
Three cells with platinum electrodes are connected in series to a DC power supply.
What is the ratio of moles formed at each cathode (negative electrode)?
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
Sub sections and their related questions
Topic 12: Atomic structure
- 16N.1.hl.TZ0.6: A period 3 element, M, forms an oxide of the type M2O. Which represents the first four successive...
-
16N.2.hl.TZ0.4d:
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
-
17M.1.hl.TZ1.5:
Which statement explains one of the decreases in first ionization energy (I.E.) across period 3?
A. The nuclear charge of element Al is greater than element Mg.
B. The electron-electron repulsion is greater, for the electron with the opposite spin, in element S than in element P.
C. A new sub-level is being filled at element S.
D. The p orbital being filled in element Al is at a lower energy than the s orbital in element Mg.
-
17M.2.hl.TZ1.2d.iii:
Sketch a graph of the first six successive ionization energies of vanadium on the axes provided.

-
17M.2.hl.TZ2.4c:
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
-
17M.2.hl.TZ1.2d.iv:
Explain why an aluminium-titanium alloy is harder than pure aluminium.
-
17N.1.hl.TZ0.6:
The graph represents the first ten ionisation energies (IE) of an element.
What is the element?
A. O
B. S
C. Ne
D. Cl
- 18M.1.hl.TZ1.5: Which transition on the diagram corresponds to the ionization of hydrogen in the ground state?
-
18M.2.hl.TZ1.2e:
Sketch a graph of the first six ionization energies of calcium.
- 18M.1.hl.TZ2.5: The graph shows the first ionization energies of some consecutive elements. Which statement is...
-
18M.2.hl.TZ2.3a.iii:
Hydrogen spectral data give the frequency of 3.28 × 1015 s−1 for its convergence limit.
Calculate the ionization energy, in J, for a single atom of hydrogen using sections 1 and 2 of the data booklet.
- 18N.1.hl.TZ0.5: The values for the first three successive ionization energies for two elements X and Z are...
- 18N.2.hl.TZ0.4c: Sketch a graph to show the relative values of the successive ionization energies of boron.
-
19M.2.hl.TZ1.3a:
Plot the relative values of the first four ionization energies of sodium.
-
19M.2.hl.TZ2.3c:
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
- 19M.1.hl.TZ1.5: Which element is represented by the first eight successive ionization energies on the...
-
19M.1.hl.TZ2.5:
Which of the following transitions in the hydrogen atom emits the least energy?
A. n = 2 to n = 1
B. n = 3 to n = 1
C. n = 4 to n = 2
D. n = 4 to n = 3
-
19N.2.hl.TZ0.1e(ii):
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
- 19N.1.hl.TZ0.5: Which shows the first ionization energies of successive elements across period 2, from left to...
- 20N.1.hl.TZ0.5: Which element is in group 13?
-
20N.2.hl.TZ0.6a:
The electron configuration of copper makes it a useful metal.
Determine the frequency of a photon that will cause the first ionization of copper. Use sections 1, 2 and 8 of the data booklet.
- 21M.1.hl.TZ1.6: The diagram shows the first ionisation energies of consecutive elements in the same period of the...
- 21M.1.hl.TZ2.5: The first eight successive ionization energies for an element are shown. In which group is the...
- 21M.2.hl.TZ1.1c: Sketch the first eight successive ionisation energies of sulfur.
-
21M.2.hl.TZ2.2a(ii):
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
- 21N.1.hl.TZ0.5: Which statement explains why the second ionization energy of aluminium is higher than the first...
-
21N.2.hl.TZ0.2b:
Sodium emits yellow light with a frequency of 5.09 × 1014 Hz when electrons transition from 3p to 3s orbitals.
Calculate the energy difference, in J, between these two orbitals using sections 1 and 2 of the data booklet.
Darling, D, n.d. D lines (of sodium). [online] Available at <https://www.daviddarling.info/encyclopedia/D/D_lines.html> [Accessed 6 May 2020].
-
22M.1.hl.TZ1.5:
The graph shows the first six ionization energies of an element.
[Ionization energies of the elements (data page) Available at: https://en.wikipedia.org/wiki/Ionization_energies_of_the_
elements_(data_page) Text is available under the Creative Commons Attribution-ShareAlike License 3.0 (CC BY-SA
3.0) https://creativecommons.org/licenses/by-sa/3.0/deed.en.]
In which group is the element?A. 13
B. 14
C. 15
D. 16
- 22M.2.hl.TZ1.1e(iv): Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization...
- 22M.2.hl.TZ2.5a(ii): Explain why there is a large increase from the 8th to the 9th ionization energy of iron.
Topic 13: The periodic table—the transition metals
-
16N.1.hl.TZ0.10:
What is the correct explanation for the colour of [Cu(H2O)6]2+?
A. Light is absorbed when an electron moves to a d orbital of higher energy.
B. Light is released when an electron moves to a d orbital of higher energy.
C. Light is absorbed when electrons move from the ligands to the central metal ion.
D. Light is absorbed when electrons move between d and s orbitals.
- 16N.2.hl.TZ0.2f: Explain how ethanedioate ions act as ligands.
-
16N.1.hl.TZ0.9:
The oxidation state of cobalt in the complex ion [Co(NH3)5Br]x is +3. Which of the following statements are correct?
I. The overall charge, x, of the complex ion is 2+.
II. The complex ion is octahedral.
III. The cobalt(III) ion has a half-filled d-subshell.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
17M.1.hl.TZ1.8:
What is the charge on the iron(III) complex ion in [Fe(OH)2(H2O)4]Br?
A. 0
B. 1+
C. 2+
D. 3+
-
17M.2.hl.TZ1.2e:
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
-
17M.2.hl.TZ1.2f:
Outline why transition metals form coloured compounds.
-
17M.2.hl.TZ1.4a:
Formulate an equation for the oxidation of nickel(II) sulfide to nickel(II) oxide.
-
17M.2.hl.TZ1.4b:
The nickel obtained from another ore, nickeliferous limonite, is contaminated with iron. Both nickel and iron react with carbon monoxide gas to form gaseous complexes, tetracarbonylnickel, , and pentacarbonyliron, . Suggest why the nickel can be separated from the iron successfully using carbon monoxide.
-
17M.2.hl.TZ1.4d:
Suggest why experiments involving tetracarbonylnickel are very hazardous.
-
17M.1.hl.TZ2.8:
Ammonia is a stronger ligand than water. Which is correct when concentrated aqueous ammonia solution is added to dilute aqueous copper(II) sulfate solution?
A. The d-orbitals in the copper ion split.
B. There is a smaller splitting of the d-orbitals.
C. Ammonia replaces water as a ligand.
D. The colour of the solution fades.
-
17M.2.hl.TZ2.2b.iii:
Explain why iron forms many different coloured complex ions.
-
17N.1.hl.TZ0.10:
[CoCl6]3– is orange while [Co(NH3)6]3+ is yellow. Which statement is correct?
A. [CoCl6]3– absorbs orange light.
B. The oxidation state of cobalt is different in each complex.
C. The different colours are due to the different charges on the complex.
D. The different ligands cause different splitting in the 3d orbitals.
- 17N.2.hl.TZ0.3d.i: State the shape of the complex ion.
- 17N.2.hl.TZ0.3d.ii: Deduce the charge on the complex ion and the oxidation state of cobalt.
- 17N.2.hl.TZ0.3e: Describe, in terms of acid-base theories, the type of reaction that takes place between the...
- 18M.1.hl.TZ1.8: Which complex has the greatest d orbital splitting?
-
18M.2.hl.TZ1.1h:
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
- 18M.1.hl.TZ2.8: Part of the spectrochemical series is shown for transition metal complexes. I−< Cl− < H2O...
-
18M.2.hl.TZ2.3d:
Explain why transition metals exhibit variable oxidation states in contrast to alkali metals.
-
18N.1.hl.TZ0.8:
Which is correct for the complex ion in [Fe(H2O)5Cl]SO4?
-
18N.2.hl.TZ0.3e:
State and explain the magnetic property of iron(II) and iron(III) ions.
-
18N.3.hl.TZ0.1c:
Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
-
19M.2.hl.TZ1.6b:
Explain why, when ligands bond to the iron ion causing the d-orbitals to split, the complex is coloured.
-
19M.2.hl.TZ2.4b(iii):
Predict two other chemical properties you would expect rhenium to have, given its position in the periodic table.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.1.hl.TZ1.8:
Which electrons are removed from iron (Z = 26) to form iron(II)?
A. two 3d electrons
B. two 4s electrons
C. one 4s electron and one 3d electron
D. two 4p electrons
- 19M.1.hl.TZ2.6: How is colour produced in transition metal complexes? A. Light is absorbed when electrons are...
-
19M.1.hl.TZ2.8:
What is the oxidation state of the metal ion and charge of the complex ion in [Co(NH3)4Cl2]Cl?
-
19N.2.hl.TZ0.6f(i):
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
-
19N.2.hl.TZ0.6f(ii):
Deduce why the Cu(I) solution is colourless.
-
19N.2.hl.TZ0.6f(iv):
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
- 19N.1.hl.TZ0.8: What is the effect of a stronger ligand?
-
20N.1.hl.TZ0.8:
Which of these statements are correct?
I. Zinc is not a transition element.
II. Ligands are Lewis bases.
III. Manganese(II) chloride is paramagnetic.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
20N.2.hl.TZ0.6b:
The electron configuration of copper makes it a useful metal.
Explain why a copper(II) solution is blue, using section 17 of the data booklet.
-
21M.1.hl.TZ1.9:
What is the overall charge, , of the chromium (III) complex?
A. 0
B. 1+
C. 2−
D. 3+
- 21M.1.hl.TZ2.8: Which factor does not affect the colour of a complex ion? A. temperature of the solution B. ...
- 21M.2.hl.TZ1.3f: Outline why, unlike typical transition metals, zinc compounds are not coloured.
- 21M.2.hl.TZ1.3g: Transition metals like iron can form complex ions. Discuss the bonding between transition metals...
-
21M.2.hl.TZ2.2b(iii):
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
-
21M.2.hl.TZ2.2b(iv):
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
-
21N.1.hl.TZ0.8:
Which complex ion contains a central ion with an oxidation state of +3?
A. [PtCl6]2−B. [Cu(H2O)4(OH)2]
C. [Ni(NH3)4(H2O)2]2+
D. [Co(NH3)4Cl2]+
-
21N.2.hl.TZ0.9b(iii):
Copper is a transition metal that forms different coloured complexes. A complex [Cu(H2O)6]2+ (aq) changes colour when excess Cl− (aq) is added.
Explain the cause of this colour change, using sections 3 and 15 from the data booklet.
-
22M.1.hl.TZ1.8:
Why is hydrated copper (II) sulfate blue?
A. Blue light is emitted when electrons return to lower d-orbitals.
B. Light complimentary to blue is absorbed when electrons return to lower d-orbitals.
C. Blue light is emitted when electrons are promoted between d-orbitals.
D. Light complimentary to blue is absorbed when electrons are promoted between d-orbitals.
- 22M.1.hl.TZ2.7: Which of these ions are likely to be paramagnetic? I. Ti3+II. Cr3+III. Fe3+ A. I and II...
-
22M.1.hl.TZ2.8:
[Cr(OH2)6]3+ is violet and [Cr(NH3)6]3+ is yellow. What is correct?
The Colour Wheel
-
22M.2.hl.TZ1.4e:
Mg(OH)+ is a complex ion, but Mg is not regarded as a transition metal. Contrast Mg with manganese, Mn, in terms of one characteristic chemical property of transition metals, other than complex ion formation.
- 22M.2.hl.TZ2.7d: Explain why transition metal cyanide complexes are coloured.
Topic 14: Chemical bonding and structure
-
16N.1.hl.TZ0.14:
Which species has bond angles of 90°?
A. AlCl4-
B. Cl4-
C. NH4+
D. SiCl4
- 16N.1.hl.TZ0.15: What is the hybridization of the numbered atoms in ethanoic acid?
-
16N.2.hl.TZ0.5b:
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
-
16N.3.hl.TZ0.22c:
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
- 17M.1.hl.TZ1.11: Which combination describes the PH4+ ion?
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.hl.TZ1.13: Which species have resonance structures? I. Ozone, O3II. Carbon dioxide, CO2III. ...
-
17M.2.hl.TZ1.5b:
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
-
17M.2.hl.TZ1.8a:
Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
-
17M.2.hl.TZ1.8b:
Suggest why the loss of ozone is an international environmental concern.
- 17M.1.hl.TZ2.10: Which does not show resonance? A. PO43– B. C6H6 C. C6H12 D. O3
- 17M.1.hl.TZ2.13: Which statement is correct? A. Sigma bonds are formed only by the combination of s atomic...
-
17M.2.hl.TZ2.3b:
Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
-
17M.2.hl.TZ2.4b.i:
Discuss the bonding in the resonance structures of ozone.
-
17M.2.hl.TZ2.4b.ii:
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
-
17M.2.hl.TZ2.7c.ii:
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
-
17M.2.hl.TZ1.5a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17M.2.hl.TZ1.5c:
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium. - 17N.1.hl.TZ0.13: What is the hybridization state and electron domain geometry around the circled C, N and...
-
17N.1.hl.TZ0.14:
How many sigma (σ) and pi (π) bonds are present in this molecule?
-
17N.2.hl.TZ0.4a:
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
- 17N.2.hl.TZ0.4c: State the type of hybridization shown by the phosphorus atom in PF3.
- 18M.1.hl.TZ1.12: Which molecules have at least one sp2 hybridized atom? I. CH3COOH II. ...
- 18M.1.hl.TZ1.13: Which can be represented with only one Lewis structure? A. CH2O B. C6H6 C. O3 D. ...
-
18M.2.hl.TZ1.1i:
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
-
18M.2.hl.TZ1.2g.i:
Describe how sigma (σ) and pi () bonds are formed.
-
18M.2.hl.TZ1.2g.ii:
Deduce the number of σ and bonds in a molecule of ethyne.
- 18M.1.hl.TZ2.12: Which molecule has an expanded octet? A. CO B. CO2 C. SF2 D. SF4
-
18M.1.hl.TZ2.13:
Which overlap of atomic orbitals leads to the formation of only a sigma (σ) bond?
I. s − p
II. p − p
III. s − s
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
18M.2.hl.TZ2.7c.i:
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.

-
18M.2.hl.TZ2.7c.ii:
Deduce, giving a reason, the more likely structure.
-
18M.2.hl.TZ2.7d:
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
-
18N.1.hl.TZ0.12:
What is the number of sigma (σ) and pi (π) bonds in the molecule (NC)2C=C(CN)2?
- 18N.1.hl.TZ0.13: What is the hybridization of the circled carbon, oxygen and nitrogen atoms?
-
18N.2.hl.TZ0.3b.i:
Draw two Lewis (electron dot) structures for BrO3−.
- 18N.2.hl.TZ0.3b.ii: Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving...
- 18N.2.hl.TZ0.6a.ii: Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
-
19M.2.hl.TZ1.2e:
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
-
19M.2.hl.TZ1.5c(ii):
State the hybridization of the nitrogen atom in chloramine.
-
19M.2.hl.TZ2.3a(ii):
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
-
19M.2.hl.TZ2.3d(i):
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
-
19M.2.hl.TZ2.3d(iii):
Deduce the hybridization of the central nitrogen atom in the molecule.
- 19M.1.hl.TZ1.12: Which species has delocalized electrons? A. OH− B. H2CO C. CO2 D. CO32−
- 19M.1.hl.TZ1.13: How many carbon atoms are sp3, sp2 and sp hybridized in the molecule?
- 19M.1.hl.TZ2.11: Which species has a square planar molecular geometry? A. SF4 B. XeF4 C. CF4 D. PF4+
-
19M.1.hl.TZ2.12:
How many sigma (σ) and pi (π) bonds are present in hydrogen cyanide, HCN?
- 19M.1.hl.TZ2.13: What is the hybridization of carbon and oxygen in methanol?
- 19N.2.hl.TZ0.1e(i): Identify the steps which absorb ultraviolet light.
- 19N.2.hl.TZ0.1f: Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor...
-
19N.2.hl.TZ0.6f(iii):
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
- 19N.1.hl.TZ0.12: Which atom is sp2 hybridized? A. C in H2CO B. C in CO2 C. N in CH3NH2 D. O in H2O
- 19N.1.hl.TZ0.13: Which atom does not obey the octet rule? A. C in CO2 B. F in BF3 C. O in H2O D. S in SF6
-
20N.1.hl.TZ0.11:
Which combination correctly describes the geometry of ?
-
20N.2.hl.TZ0.1e(iii):
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
-
20N.2.hl.TZ0.2b:
State the type of hybridization shown by the central carbon atom in molecule B.
-
20N.2.hl.TZ0.2c:
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
- 21M.1.hl.TZ1.12: Which contain delocalised electrons? I. C6H5OHII. CH3COO−III. CO32− A. I and II only B. I...
- 21M.1.hl.TZ1.13: In which series are all carbon atoms sp2 hybridized? A. C2H2 H2CO HCOOH B. C2H4 ...
- 21M.1.hl.TZ2.13: What is the electron domain geometry of Si in SiO2? A. bent B. linear C. square planar D. ...
- 21M.2.hl.TZ2.4c: State the hybridization of the carbon I and II atoms in but-2-ene.
-
21M.2.hl.TZ2.4d:
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
-
21N.1.hl.TZ0.12:
Which molecules contain two pi () bonds?
I. HCN
II. H2CO3
III. H2C2O4
A. I and II onlyB. I and III only
C. II and III only
D. I, II and III
- 21N.1.hl.TZ0.13: What is the hybridization of nitrogen and chlorine in NCl3?
- 21N.2.hl.TZ0.3b(ii): Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
-
21N.2.hl.TZ0.10a(i):
Distinguish between a sigma and pi bond.
- 21N.2.hl.TZ0.10a(ii): Identify the hybridization of carbon in ethane, ethene and ethyne.
- 22M.1.hl.TZ1.12: In which compound are all carbon atoms sp3 hybridized? A. C2H2 B. C2H2Cl2 C. C2Cl4 D. C2Cl6
-
22M.1.hl.TZ2.11:
What is the formal charge of the oxygen atom in H3O+?
A. −2
B. −1
C. 0
D. +1
- 22M.1.hl.TZ2.12: What is the molecular geometry of SF4? A. Tetrahedral B. Trigonal bipyramidal C. ...
-
22M.2.hl.TZ1.5a(iii):
State the number of (sigma) and (pi) bonds in Compound A.
- 22M.2.hl.TZ1.5a(iv): Deduce the hybridization of the central carbon atom in Compound A.
-
22M.2.hl.TZ1.6a(ii):
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
-
22M.2.hl.TZ1.6a(iii):
Explain the relative lengths of the three bonds between N and O in nitric acid.
-
22M.2.hl.TZ2.7b(i):
Sketch the shape of one sigma () and one pi () bond.
-
22M.2.hl.TZ2.7b(ii):
Identify the number of sigma and pi bonds in HCN.
- 22M.2.hl.TZ2.7b(iii): State the hybridization of the carbon atom in HCN.
Topic 15: Energetics/thermochemistry
- 16N.1.hl.TZ0.18: Which represents the enthalpy change of hydration of the chloride ion?
- 16N.1.hl.TZ0.19: Which ionic compound has the largest value of lattice enthalpy? A. MgS B. MgO C. CaBr2 D. NaF
-
16N.2.hl.TZ0.1b:
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
-
17M.1.hl.TZ1.16:
Which equation represents enthalpy of hydration?
A. Na(g) → Na+(aq) + e−
B. Na+(g) → Na+(aq)
C. NaCl(s) → Na+(g) + Cl−(g)
D. NaCl(s) → Na+(aq) + Cl−(aq)
- 17M.1.hl.TZ1.17: Which combination of ΔH θ and ΔS θ will result in a non-spontaneous reaction at all temperatures?
-
17M.2.hl.TZ1.3c.ii:
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
-
17M.2.hl.TZ1.4c.i:
Calculate the standard entropy change, , of the reaction, in , using the values given.
-
17M.2.hl.TZ1.4c.ii:
Calculate a value for in kJ.
-
17M.2.hl.TZ1.4c.iii:
Use your answers to (c)(i) and (c)(ii), to determine the temperature, in °C, at which the decomposition of liquid tetracarbonylnickel to nickel and carbon monoxide becomes favourable.
(If you did not get answers to (c)(i) and (c)(ii), use and respectively but these are not the correct answers.) -
17M.1.hl.TZ2.16:
The Born-Haber cycle for potassium oxide is shown below:
Which expression represents the lattice enthalpy in kJ mol–1?
A. –361 + 428 + 838 + 612
B. –(–361) + 428 + 838 + 612
C. –361 + 428 + 838 – 612
D. –(–361) + 428 + 838 – 612
- 17M.1.hl.TZ2.17: Which ion’s hydration energy is the most exothermic? A. Li+ B. Na+ C. Br– D. I–
-
17M.2.hl.TZ2.9b.i:
Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for the hydrogenation of propene.
-
17M.2.hl.TZ2.9b.ii:
The standard enthalpy change, ΔH θ, for the hydrogenation of propene is –124.4 kJ mol–1. Predict the temperature above which the hydrogenation reaction is not spontaneous.
- 17N.1.hl.TZ0.15: Which statements are correct for ionic compounds? I. Lattice energy increases as ionic radii...
-
17N.1.hl.TZ0.16:
What is the standard enthalpy of formation, in kJ mol–1, of IF (g)?
IF7 (g) + I2 (s) → IF5 (g) + 2IF (g) ΔH = –89 kJ
ΔH (IF7) = –941 kJ mol–1
ΔH (IF5) = –840 kJ mol–1
A. –190
B. –95
C. +6
D. +95
-
17N.1.hl.TZ0.17:
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
-
17N.1.hl.TZ0.18:
Which equation represents the lattice enthalpy of magnesium sulfide?
A. MgS (s) → Mg (g) + S (g)
B. MgS (s) → Mg+ (g) + S– (g)
C. MgS (s) → Mg2+ (g) + S2– (g)
D. MgS (s) → Mg (s) + S (s)
-
17N.2.hl.TZ0.5b:
Calculate the standard entropy change for this reaction using the following data.
-
17N.2.hl.TZ0.5c:
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
-
18M.1.hl.TZ1.16:
What is the enthalpy of solution of MgF2(s) in kJ mol−1?
Lattice enthalpy of MgF2(s) = 2926 kJ mol−1
Hydration enthalpy of Mg2+(g) = −1963 kJ mol−1
Hydration enthalpy of F−(g) = −504 kJ mol−1
A. 2926 − 1963 + 2(−504)
B. 2926 − 1963 − 504
C. −2926 − (−1963) − (−504)
D. −2926 − (−1963) − 2(−504)
- 18M.1.hl.TZ1.17: Which statement is correct? A. If ΔH < 0, reaction is always spontaneous. B. If ΔH...
-
18M.2.hl.TZ1.3c.iv:
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
-
18M.2.hl.TZ1.3c.v:
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
-
18M.1.hl.TZ2.16:
Which value represents the lattice enthalpy, in kJ mol−1, of strontium chloride, SrCl2?

A. – (–829) + 164 + 243 + 550 + 1064 – (–698)
B. –829 + 164 + 243 + 550 + 1064 – 698
C. – (–829) + 164 + 243 + 550 + 1064 – 698
D. –829 + 164 + 243 + 550 + 1064 – (–698)
-
18M.1.hl.TZ2.17:
Which system has the most negative entropy change, ΔS, for the forward reaction?
A. N2(g) + 3H2(g) 2NH3(g)
B. CaCO3(s) → CaO(s) + CO2(g)
C. 2S2O32−(aq) + I2(aq) → S4O62−(aq) + 2I–(aq)
D. H2O(l) → H2O(g)
-
18M.2.hl.TZ2.5c:
The table lists standard entropy, SΘ, values.

Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
-
18M.2.hl.TZ2.5d:
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
-
18M.2.hl.TZ2.5e:
Determine the temperature, in K, above which the reaction becomes spontaneous.
-
18N.1.hl.TZ0.16:
What are the signs of ΔHΘ and ΔSΘ for the reaction, which is spontaneous at low temperature and non-spontaneous at very high temperature?
ΔGΘ = ΔHΘ − TΔSΘ
SO3 (g) + CaO (s) → CaSO4 (s)
-
18N.1.hl.TZ0.17:
Which change is exothermic?
A. Cl2 (g) → Cl (g)
B. K (g) → K+ (g) + e−
C. KCl (s) → K+ (g) + Cl− (g)
D. Cl (g) + e− → Cl− (g)
- 18N.2.hl.TZ0.4d: Predict, giving your reasons, whether Mn2+ or Fe2+ is likely to have a more exothermic enthalpy...
- 18N.2.hl.TZ0.5b: Predict, giving your reason, the sign of the standard entropy change of the forward reaction.
-
18N.2.hl.TZ0.5d:
Predict, giving your reasons, whether the forward reaction is endothermic or exothermic. Use your answers to (b) and (c).
-
19M.2.hl.TZ1.3d(i):
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1O2(g) → O2- (g):
Na (s) → Na+ (g):
-
19M.2.hl.TZ1.3d(ii):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.) -
19M.2.hl.TZ1.3d(iii):
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
-
19M.2.hl.TZ2.2g(i):
Determine the standard entropy change, in J K−1, for the decomposition of dinitrogen monoxide.
2N2O (g) → 2N2 (g) + O2 (g)
-
19M.2.hl.TZ2.2g(ii):
Dinitrogen monoxide has a positive standard enthalpy of formation, ΔHfθ.
Deduce, giving reasons, whether altering the temperature would change the spontaneity of the decomposition reaction.
-
19M.1.hl.TZ1.16:
Which is correct for the reaction H2O (g) → H2O (l) ?
A. Enthalpy increases and entropy increases.
B. Enthalpy decreases and entropy increases.
C. Enthalpy increases and entropy decreases.
D. Enthalpy decreases and entropy decreases.
-
19M.1.hl.TZ1.17:
Which equation represents the standard enthalpy of atomization of bromine, Br2?
A. Br2 (l) → Br (g)
B. Br2 (l) → 2Br (g)
C. Br2 (l) → 2Br (l)
D. Br2 (l) → Br (l)
-
19M.1.hl.TZ2.16:
Which equation represents lattice enthalpy?
A. NaCl (g) → Na+ (g) + Cl− (g)
B. NaCl (s) → Na+ (g) + Cl− (g)
C. NaCl (s) → Na+ (aq) + Cl− (aq)
D. NaCl (s) → Na+ (s) + Cl− (s)
-
19M.1.hl.TZ2.17:
Which change has the greatest increase in entropy?
A. CO2 (s) → CO2 (g)
B. CO2 (g) → CO2 (l)
C. CO2 (g) → CO2 (s)
D. CO2 (l) → CO2 (s)
-
19N.2.hl.TZ0.4a(iv):
Calculate the standard Gibbs free energy change, , in kJ mol−1, for the first dissociation of citric acid at 298 K, using section 1 of the data booklet.
-
19N.2.hl.TZ0.4a(v):
Comment on the spontaneity of the reaction at 298 K.
-
19N.2.hl.TZ0.6d:
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
- 19N.2.hl.TZ0.6e(v): Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the...
-
19N.2.hl.TZ0.6e(vi):
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
-
19N.1.hl.TZ0.17:
Which reaction has the greatest increase in entropy of the system?
A. HCl (g) + NH3 (g) → NH4Cl (s)
B. (NH4)2Cr2O7 (s) → Cr2O3 (s) + N2 (g) + 4H2O (g)
C. CaCO3 (s) → CaO (s) + CO2 (g)
D. I2 (g) → I2 (s)
-
19N.1.hl.TZ0.18:
What is the order of increasing (more exothermic) enthalpy of hydration?
Xn+ (g) → Xn+ (aq)
A. Ca2+, Mg2+, K+, Na+
B. Na+, K+, Mg2+, Ca2+
C. K+, Na+, Ca2+, Mg2+
D. Mg2+, Ca2+, Na+, K+
-
20N.1.hl.TZ0.16:
Which combination gives the standard hydration enthalpy of ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.17:
Which reaction becomes more spontaneous as temperature increases?
A.
B.
C.
D.
-
20N.2.hl.TZ0.2f(ii):
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
-
20N.2.hl.TZ0.3c:
Predict, giving a reason, whether the entropy change, , for this reaction is negative or positive.
-
20N.2.hl.TZ0.3d:
Calculate for the reaction in , using section 12 of the data booklet.
The standard molar entropy for oxygen gas is .
-
20N.2.hl.TZ0.3e:
Calculate the standard Gibbs free energy change, , in , for the reaction at 5 °C, using your answers to (b) and (d). Use section 1 of the data booklet.
(If you did not obtain an answer to (b) or (d) use values of and respectively, although these are not the correct answers.)
-
21M.1.hl.TZ1.16:
The table shows the variation of standard Gibbs energy with temperature for a reversible reaction.
What can be concluded about the reaction?
A. Equilibrium shifts left as temperature increases.
B. The forward reaction is more spontaneous below 300 K.
C. Entropy is higher in the products than in the reactants.
D. Kc decreases as temperature increases.
-
21M.1.hl.TZ1.17:
Which substance has the highest lattice enthalpy?
A.
B.
C.
D.
-
21M.1.hl.TZ2.16:
Which represents electron affinity?
A. Al2+ (g) → Al3+ (g) + e−
B. C (g) + e− → C− (g)
C. Cl2 (g) → 2Cl (g)
D. S (s) → S+ (g) + e−
-
21M.1.hl.TZ2.17:
Which change results in the largest negative value of ΔS?
A. C2H5OH (l) + SOCl2 (l) → C2H5Cl (l) + SO2 (g) + HCl (g)
B. CaCO3 (s) → CaO (s) + CO2 (g)
C. H2O (l) → H2O (s)
D. NH3 (g) + HCl (g) → NH4Cl (s)
-
21M.2.hl.TZ1.4e(ii):
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
- 21M.2.hl.TZ1.4e(iii): Justify the sign of ΔS with reference to the equation.
- 21M.2.hl.TZ1.4e(iv): Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the...
-
21M.2.hl.TZ2.1b(ii):
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
-
21M.2.hl.TZ2.1b(iii):
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
-
21M.2.hl.TZ2.5a(ii):
Deduce the change in enthalpy, ΔH, in kJ, when 56.00 g of ethanol is burned. Use section 13 in the data booklet.
-
21N.1.hl.TZ0.16:
Consider the Born–Haber cycle for the formation of sodium oxide:
What is the lattice enthalpy, in kJ mol−1, of sodium oxide?
A. 414 + 2(108) + 249 + 2(496) − 141 + 790B. 414 + 2(108) + 249 + 2(496) + 141 + 790
C. −414 + 2(108) + 249 + 2(496) − 141 + 790
D. −414 − 2(108) − 249 − 2(496) + 141 − 790
- 21N.1.hl.TZ0.17: In which of the following situations is the forward reaction spontaneous? A. The equilibrium...
-
21N.2.hl.TZ0.3c(ii):
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
-
21N.2.hl.TZ0.3c(iii):
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
- 22M.1.hl.TZ1.16: Which compound has the largest value of lattice enthalpy? A. Na2O B. K2O C. Na2S D. K2S
-
22M.1.hl.TZ1.17:
In which reaction does entropy decrease?
A. NaCl (s) → NaCl (aq)
B. Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
C. NH3 (g) + HCl (g) → NH4Cl (s)
D. CuCO3 (s) → CuO (s) + CO2 (g)
-
22M.1.hl.TZ2.14:
Which equation represents hydration enthalpy?
A. Na+ (g) → Na+ (aq)
B. Na+ (aq) → Na+ (g)
C. NaCl (s) → NaCl (aq)
D. NaCl (aq) → NaCl (s)
- 22M.1.hl.TZ2.15: What are the signs of ΔH and ΔS for a reaction that is non-spontaneous at low temperatures but...
- 22M.1.hl.TZ2.17: Which term in the expression ΔG⦵ = ΔH⦵ − TΔS⦵ is an indirect measure of the entropy change of the...
-
22M.2.hl.TZ1.3c(iii):
Calculate the entropy change for the Haber–Bosch process, in J mol–1 K–1 at 298 K. Use your answer to (b)(i) and section 1 of the data booklet.
- 22M.2.hl.TZ1.3c(iv): Outline, with reference to the reaction equation, why this sign for the entropy change is expected.
-
22M.2.hl.TZ2.4d(i):
Calculate the entropy change of reaction, ΔS⦵, in J K−1 mol−1.
-
22M.2.hl.TZ2.4d(ii):
Predict, giving a reason, how the value of the ΔS⦵reaction would be affected if (g) were used as a reactant.
-
22M.2.hl.TZ2.4d(iii):
Calculate the Gibbs free energy change, ΔG⦵, in kJ mol−1, for the reaction at 298 K. Use section 1 of the data booklet.
Topic 16: Chemical kinetics
- 16N.1.hl.TZ0.21: Which statement describes the characteristics of a transition state relative to the potential...
-
16N.1.hl.TZ0.22:
Decomposition of hydrogen peroxide in an aqueous solution proceeds as follows.
2H2O2(aq) → 2H2O(l) + O2(g)
The rate expression for the reaction was found to be: rate = k [H2O2].
Which graph is consistent with the given rate expression?
-
16N.2.hl.TZ0.3e:
(i) Using the graph, explain the order of reaction with respect to sodium thiosulfate.
(ii) In a different experiment, this reaction was found to be first order with respect to hydrochloric acid. Deduce the overall rate expression for the reaction.
-
17M.1.hl.TZ1.20:
The table gives rate data for the reaction in a suitable solvent.
C4H9Br + OH− → C4H9OH + Br−
Which statement is correct?
A. The rate expression is rate = k [C4H9Br] [OH− ].
B. The rate increases by a factor of 4 when the [OH− ] is doubled.
C. C4H9Br is a primary halogenoalkane.
D. The reaction occurs via SN1 mechanism.
-
17M.1.hl.TZ1.21:
What are the units for the rate constant, k, in the expression?
Rate = k [X]2[Y]
A. mol2 dm−6 s−1
B. mol−1 dm3 s−1
C. mol dm−3 s−1
D. mol−2 dm6 s−1
-
17M.2.hl.TZ1.1b:
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
-
17M.2.hl.TZ1.1c:
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
-
17M.2.hl.TZ1.1d:
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
- 17M.1.hl.TZ2.12: Which is the first step in the CFC-catalysed destruction of ozone in UV light? A. CCl2F2 →...
-
17M.1.hl.TZ2.20:
Which is true of an Arrhenius plot of (y-axis) against ?
A. The graph goes through the origin.
B. The activation energy can be determined from the gradient.
C. The intercept on the x-axis is the activation energy.
D. The intercept on the y-axis is the frequency factor, A.
- 17M.1.hl.TZ2.21: Which is correct about reaction mechanisms? A. A species that is zero order does not take...
-
17M.2.hl.TZ2.5b.i:
State the rate expression for the reaction.
-
17M.2.hl.TZ2.5b.ii:
The following mechanism is proposed for the reaction.
Identify the rate determining step giving your reason.
-
17M.2.hl.TZ2.5b.iii:
State one method that can be used to measure the rate for this reaction.
-
17M.2.hl.TZ2.5b.v:
Sketch the relationship between the rate of reaction and the concentration of NO2.
-
17M.2.hl.TZ2.5c:
The Arrhenius equation, , gives the relationship between the rate constant and temperature.
State how temperature affects activation energy.
-
17N.1.hl.TZ0.21:
The rate expression for the reaction X (g) + 2Y (g) → 3Z (g) is
rate = k[X]0 [Y]2
By which factor will the rate of reaction increase when the concentrations of X and Y are both increased by a factor of 3?
A. 6
B. 9
C. 18
D. 27
- 17N.1.hl.TZ0.22: Which pair of statements explains the increase in rate of reaction when the temperature...
- 17N.2.hl.TZ0.2e.i: Deduce the order of reaction with respect to Cl2 and NO.
-
17N.2.hl.TZ0.2e.ii:
State the rate expression for the reaction.
- 17N.2.hl.TZ0.2e.iii: Calculate the value of the rate constant at 263 K.
- 18M.1.hl.TZ1.19: What are correct labels for the Maxwell−Boltzmann energy distribution curves?
- 18M.1.hl.TZ1.20: The reaction between NO2 and F2 gives the following rate data at a certain temperature. What...
- 18M.1.hl.TZ1.21: What is the effect of increasing temperature on the rate constant, k? A. The rate constant...
-
18M.2.hl.TZ1.4b.iv:
Deduce the rate expression for the reaction.
-
18M.2.hl.TZ1.4b.v:
Calculate the rate constant of the reaction, stating its units.
-
18M.2.hl.TZ1.4d:
Describe how the activation energy of this reaction could be determined.
- 18M.1.hl.TZ2.20: When X reacts with Y to give Z, the following graph is plotted. What can be deduced from the...
- 18M.1.hl.TZ2.21: Which statement is correct? A. The value of the rate constant, k, is independent of...
-
18M.2.hl.TZ2.6d:
The rate constant for a reaction doubles when the temperature is increased from 25.0 °C to 35 °C.
Calculate the activation energy, Ea, in kJ mol−1 for the reaction using section 1 and 2 of the data booklet.
- 18N.1.hl.TZ0.20: Compounds X and Y were mixed and the time taken for a colour to appear was recorded at various...
-
18N.1.hl.TZ0.21:
The rate expression for the reaction is: rate = k [NO]2[O2].
2NO (g) + O2 (g) → 2NO2 (g)
Which mechanism is not consistent with this rate expression?
- 18N.2.hl.TZ0.10a: Classify substances B and D as reactant, product, catalyst, or intermediate, based on the...
-
18N.2.hl.TZ0.10b:
Deduce the rate expression.
- 18N.2.hl.TZ0.10c: Calculate the initial rate of reaction for experiment 2, if measured under the same conditions.
-
19M.2.hl.TZ1.4b(ii):
Two more trials (2 and 3) were carried out. The results are given below.
Determine the rate equation for the reaction and its overall order, using your answer from (b)(i).
Rate equation:
Overall order:
-
19M.2.hl.TZ2.2c(i):
Deduce how the rate of reaction at t = 2 would compare to the initial rate.
-
19M.2.hl.TZ2.2c(ii):
It has been suggested that the reaction occurs as a two-step process:
Step 1: N2O (g) → N2 (g) + O (g)
Step 2: N2O (g) + O (g) → N2 (g) + O2 (g)
Explain how this could support the observed rate expression.
- 19M.1.hl.TZ1.20: Which graph is obtained from a first order reaction?
- 19M.1.hl.TZ1.21: Which is correct for the reaction mechanism shown?
-
19M.1.hl.TZ2.20:
Which statement is correct about a catalyst?
A. It decreases the activation energy of the forward reaction but not the reverse.
B. It increases the proportion of products to reactants in an equilibrium.
C. It decreases the enthalpy change of the reaction.
D. It changes the mechanism of the reaction.
-
19M.1.hl.TZ2.21:
What is the order with respect to each reactant?
2NO (g) + Cl2 (g) → 2NOCl (g)
- 19N.2.hl.TZ0.1e(i): Identify the steps which absorb ultraviolet light.
-
19N.2.hl.TZ0.3b(i):
Determine the rate expression from the results, explaining your method.
- 19N.1.hl.TZ0.21: Which is correct?
-
19N.1.hl.TZ0.22:
What is the intercept on the y-axis when a graph of lnk is plotted against on the x-axis?
A. lnA
B.
C.
D.
-
20N.1.hl.TZ0.20:
What are the units of the rate constant, , if the rate equation is ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.21:
Which graph represents the relationship between the rate constant, , and temperature, , in kelvin?
-
21M.1.hl.TZ1.20:
A reaction proceeds by the following mechanism:
step 1:
step 2:Which rate equation is consistent with this mechanism?
A. Rate = k[B]2[C]
B. Rate = k[A]2[B][C]
C. Rate = k[A]2
D. Rate = k[A][C]
- 21M.1.hl.TZ1.21: Which graphs show a first order reaction? A. V and X B. V and Y C. W and X D. W and Y
- 21M.1.hl.TZ2.20: Which graph represents a second order reaction with respect to X? X → Y
-
21M.2.hl.TZ1.6b(iii):
Write the rate expression for this reaction.
-
21M.2.hl.TZ1.6b(iv):
Calculate the value of the rate constant, k, giving its units.
-
21M.2.hl.TZ1.7c:
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
-
21M.2.hl.TZ2.6a:
Determine the rate expression for the reaction.
-
21M.2.hl.TZ2.6b:
Determine the value and unit of the rate constant using the rate expression in (a).
- 21N.1.hl.TZ0.20: Which graph shows a first order reaction?
-
21N.1.hl.TZ0.21:
The rate equation for a reaction is:
rate = k[A][B]
Which mechanism is consistent with this rate equation?
A. 2A I Fast
I + B → P SlowB. A + B I Fast
I + A → P SlowC. A → I Slow
I + B → P FastD. B I Fast
I + A → P Slow -
21N.2.hl.TZ0.10c(i):
Deduce the rate expression for this reaction.
- 21N.2.hl.TZ0.10c(ii): Deduce the units of the rate constant.
-
21N.2.hl.TZ0.10c(iii):
Determine the initial rate of reaction in experiment 4.
- 21N.2.hl.TZ0.10d: Deduce, with a reason, the mechanism of the reaction between 2-chloropentane and sodium hydroxide.
- 22M.1.hl.TZ1.20: The table shows data for the hydrolysis of a halogenoalkane, RCl. Which statements are...
-
22M.1.hl.TZ1.21:
What is the activation energy according to the following plot of the linear form of the Arrhenius equation?
Arrhenius equation: .
A.
B.
C.
D.
- 22M.1.hl.TZ2.20: Which energy profile diagram represents an exothermic SN1 reaction?
-
22M.2.hl.TZ1.2c(i):
Use the graph to deduce the dependence of the reaction rate on the amount of Mg.
-
22M.2.hl.TZ1.2c(ii):
The reaction is first order with respect to HCl. Calculate the time taken, in seconds (s), for half of the Mg to dissolve when [HCl] = 0.5 mol dm–3.
-
22M.2.hl.TZ2.4a(i):
Deduce the order of reaction with respect to hydrogen.
-
22M.2.hl.TZ2.4a(ii):
Deduce the rate expression for the reaction.
-
22M.2.hl.TZ2.4a(iii):
Calculate the value of the rate constant stating its units.
Topic 17: Equilibrium
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and respectively.
X + 2Y 2W + Z
What is the value of the equilibrium constant, Kc?
A.
B.
C. 2
D. 8
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
-
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?

-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
Topic 18: Acids and bases
-
16N.1.hl.TZ0.28:
Which mixture is a buffer solution?
A. 25 cm3 of 0.10 mol dm-3 NH3 (aq) and 50 cm3 of 0.10 mol dm-3 HCl (aq)
B. 50 cm3 of 0.10 mol dm-3 NH3 (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
C. 25 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
D. 50 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
-
16N.1.hl.TZ0.29:
Which salt solution has the highest pH?
A. NH4Cl
B. Ca(NO3)2
C. Na2CO3
D. K2SO4
-
16N.2.hl.TZ0.7a:
Calculate the pH of 0.0100 mol dm–3 methanoic acid stating any assumption you make. Ka = 1.6 × 10–4.
-
16N.2.hl.TZ0.7b:
(i) Sketch a graph of pH against volume of a strong base added to a weak acid showing how you would determine pKa for the weak acid.
(ii) Explain, using an equation, why the pH increases very little in the buffer region when a small amount of alkali is added.
-
17M.1.hl.TZ1.27:
A buffer is produced by mixing 20.0 cm3 of 0.10 mol dm−3 ethanoic acid, CH3COOH(aq), with 0.10 mol dm−3 sodium hydroxide, NaOH(aq).
What is the volume of NaOH required and the pH of the buffer?
-
17M.2.hl.TZ1.2e:
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
-
17M.2.hl.TZ1.5d.i:
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
-
17M.2.hl.TZ1.5d.ii:
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
- 17M.1.hl.TZ2.26: Which type of bond is formed when a Lewis acid reacts with a Lewis base? A. Covalent B. ...
- 17M.1.hl.TZ2.27: What is the order of increasing acidity of the following acids? A. chloroethanoic <...
-
17M.2.hl.TZ2.8b.iv:
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
-
17M.2.hl.TZ2.8b.vi:
Deduce the pKa for this acid.
-
17M.2.hl.TZ2.8c:
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
- 17N.1.hl.TZ0.26: Which of the following will form a buffer solution if combined in appropriate molar ratios? A....
-
17N.1.hl.TZ0.27:
Which indicator is appropriate for the acid-base titration shown below?
A. Thymol blue (pKa = 1.5)
B. Methyl orange (pKa = 3.7)
C. Bromophenol blue (pKa = 4.2)
D. Phenolphthalein (pKa = 9.6) - 17N.2.hl.TZ0.3e: Describe, in terms of acid-base theories, the type of reaction that takes place between the...
-
17N.2.hl.TZ0.6c.i:
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
-
17N.2.hl.TZ0.6c.ii:
Calculate Kb for HCO3– acting as a base.
- 18M.1.hl.TZ1.26: Which statements are correct? I. Lewis bases can act as nucleophiles. II....
- 18M.1.hl.TZ1.27: Which combination of acid and base is most likely to have a pH of 8.5 at the equivalence point in...
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
-
18M.2.hl.TZ1.5d:
Determine the pH of 0.010 mol dm−3 2,2-dimethylpropanoic acid solution.
Ka (2,2-dimethylpropanoic acid) = 9.333 × 10−6
-
18M.2.hl.TZ1.5e:
Explain, using appropriate equations, how a suitably concentrated solution formed by the partial neutralization of 2,2-dimethylpropanoic acid with sodium hydroxide acts as a buffer solution.
- 18M.1.hl.TZ2.26: Which is an example of a Lewis base? A. an electrophile B. BF3 C. CH4 D. a...
- 18M.1.hl.TZ2.27: What is the order of increasing acidity? A. HClO < CH3CH2COOH < HF < HIO3 B. ...
-
18M.2.hl.TZ2.2d.i:
The graph represents the titration of 25.00 cm3 of 0.100 mol dm−3 aqueous ethanoic acid with 0.100 mol dm−3 aqueous sodium hydroxide.

Deduce the major species, other than water and sodium ions, present at points A and B during the titration.
-
18M.2.hl.TZ2.2d.ii:
Calculate the pH of 0.100 mol dm−3 aqueous ethanoic acid.
Ka = 1.74 × 10−5
-
18M.2.hl.TZ2.2d.iv:
Predict whether the pH of an aqueous solution of ammonium chloride will be greater than, equal to or less than 7 at 298 K.
- 18N.1.hl.TZ0.25: What is the order of increasing pH for the following solutions of the same concentration? A. ...
- 18N.1.hl.TZ0.26: Which species is not a Lewis base? A. OH− B. NH4+ C. H2O D. PH3
-
18N.1.hl.TZ0.27:
An indicator, HIn, has a pKa of 5.1.
HIn (aq) H+ (aq) + In− (aq)
colour A colour B
Which statement is correct?
A. At pH = 7, colour B would be observed
B. At pH = 3, colour B would be observed
C. At pH = 7, [HIn] = [In−]
D. At pH = 3, [HIn] < [In−] -
18N.2.hl.TZ0.6b.ii:
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
-
18N.2.hl.TZ0.6c:
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
- 18N.2.hl.TZ0.9d: State, giving your reason, whether the hydroxide ion acts as a Lewis acid, a Lewis base, or...
-
19M.2.hl.TZ1.5d(i):
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
-
19M.2.hl.TZ1.5d(ii):
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
-
19M.2.hl.TZ1.5d(iii):
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
-
19M.2.hl.TZ2.5c:
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
-
19M.2.hl.TZ2.5f:
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
- 19M.1.hl.TZ1.26: Which is a Lewis acid but not a Brønsted−Lowry acid? A. AlCl3 B. CH3CO2H C. HF D. CCl4
-
19M.1.hl.TZ1.27:
Which has the strongest conjugate base?
A. HCOOH (Ka = 1.8 × 10−4)
B. HNO2 (Ka = 7.2 × 10−4)
C. HCN (Ka = 6.2 × 10−10)
D. HIO3 (Ka = 1.7 × 10−1)
-
19M.1.hl.TZ2.26:
Where is the buffer region for the titration of a weak acid with a strong base?
-
19M.1.hl.TZ2.27:
The following equation represents the dissociation of water at 25 °C.
2H2O (l) H3O+ (aq) + OH− (aq) ΔH = +56 kJ
Which changes occur as the temperature increases?
A. [H3O+] increases and pH will decrease.
B. [H3O+] decreases and pH will increase.
C. [H3O+] increases and pH will increase.
D. [H3O+] decreases and pH will decrease.
- 19N.2.hl.TZ0.5a: A sample of ethanoic acid was titrated with sodium hydroxide solution, and the following pH curve...
- 19N.2.hl.TZ0.5b(i): Identify the most suitable indicator for the titration using section 22 of the data booklet.
-
19N.2.hl.TZ0.5b(ii):
Describe, using a suitable equation, how the buffer solution formed during the titration resists pH changes when a small amount of acid is added.
-
19N.2.hl.TZ0.6f(iv):
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.1.hl.TZ0.27: Which can act as a Lewis acid but not a Brønsted–Lowry acid? A. BF3 B. H2O C. NF3 D. NH3
- 19N.1.hl.TZ0.28: What is the order, in increasing pH, of the following solutions of equal concentration? A....
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
-
20N.2.hl.TZ0.5b(i):
Identify the major species, other than water and potassium ions, at these points.
- 20N.2.hl.TZ0.5b(ii): State a suitable indicator for this titration. Use section 22 of the data booklet
-
20N.2.hl.TZ0.5b(iii):
Suggest, giving a reason, which point on the curve is considered a buffer region.
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
20N.2.hl.TZ0.5e:
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
- 21M.1.sl.TZ1.19: Which is amphiprotic? A. NH4+ B. PO43− C. H2O D. H3O+
- 21M.1.hl.TZ1.27: Which combination will produce an alkaline buffer in water? A. 0.10 mol NH3 and 0.05 mol...
- 21M.1.hl.TZ2.26: Which is correct? A. Electrophiles are Brønsted–Lowry acids. B. Nucleophiles are...
- 21M.1.hl.TZ2.27: Which compound is acidic in aqueous solution? A. KBr B. CH3COONa C. NH4Cl D. Na2CO3
- 21M.2.hl.TZ1.3g: Transition metals like iron can form complex ions. Discuss the bonding between transition metals...
-
21M.2.hl.TZ1.8a:
Calculate the pH of 0.00100 mol dm–3 propanoic acid solution. Use section 21 of the data booklet.
-
21M.2.hl.TZ1.8b:
Sketch the general shape of the variation of pH when 50 cm3 of 0.001 mol dm–3 NaOH (aq) is gradually added to 25 cm3 of 0.001 mol dm–3 CH3CH2COOH (aq).
- 21M.2.hl.TZ2.5d(i): Sketch the titration curve of methanoic acid with sodium hydroxide, showing how you would...
-
21M.2.hl.TZ2.5d(ii):
Identify an indicator that could be used for the titration in 5(d)(i), using section 22 of the data booklet.
-
21M.2.hl.TZ2.5e:
Determine the concentration of methanoic acid in a solution of pH = 4.12. Use section 21 of the data booklet.
-
21M.2.hl.TZ2.5f:
Identify if aqueous solutions of the following salts are acidic, basic, or neutral.
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
- 21N.2.hl.TZ0.10e: Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
-
21N.2.hl.TZ0.11a:
Calculate the initial pH before any sodium hydroxide was added, using section 21 of the data booklet.
-
21N.2.hl.TZ0.11b:
The concentration of excess sodium hydroxide was 0.362 mol dm−3. Calculate the pH of the solution at the end of the experiment.
-
21N.2.hl.TZ0.11c:
Sketch the neutralisation curve obtained and label the equivalence point.
- 22M.1.hl.TZ1.26: Which statement explains the Lewis acid–base nature of the chloride ion in this reaction? C2H5+...
- 22M.1.hl.TZ1.27: In which set are the salts arranged in order of increasing pH? A. HCOONH4 < KBr < NH4Br...
- 22M.1.hl.TZ2.26: A weak base is titrated with a strong acid. Which value of pKb can be estimated from this...
- 22M.1.hl.TZ2.27: Which species are both Lewis and Brønsted–Lowry bases? I. CN−II. OH−III. NH3 A. I and II...
- 22M.2.hl.TZ1.1d(ii): Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that...
-
22M.2.hl.TZ1.4c(ii):
Calculate the concentration, in mol dm–3, of ammonia molecules in the solution with pH = 9.3. Use section 21 of the data booklet.
-
22M.2.hl.TZ1.4d:
Magnesium salts form slightly acidic solutions owing to equilibria such as:
Mg2+ (aq) + H2O (l) Mg(OH)+ (aq) + H+ (aq)
Comment on the role of Mg2+ in forming the Mg(OH)+ ion, in acid-base terms.
- 22M.2.hl.TZ2.7a(i): State why NH3 is a Lewis base.
-
22M.2.hl.TZ2.7a(ii):
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
- 22M.2.hl.TZ2.7a(iii): Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer...
-
22M.2.hl.TZ1.4c(iii):
An aqueous solution containing high concentrations of both NH3 and NH4+ acts as an acid-base buffer solution as a result of the equilibrium:
NH3 (aq) + H+ (aq) NH4+ (aq)
Referring to this equilibrium, outline why adding a small volume of strong acid would leave the pH of the buffer solution almost unchanged.
Topic 19: Redox processes
- 16N.1.hl.TZ0.32: Which signs for both Eθcell and ΔGθ result in a spontaneous redox reaction occurring under...
-
16N.1.hl.TZ0.33:
An iron rod is electroplated with silver. Which is a correct condition for this process?
A. The silver electrode is the positive electrode.
B. The iron rod is the positive electrode.
C. The electrolyte is iron(II) sulfate.
D. Oxidation occurs at the negative electrode.
-
16N.2.hl.TZ0.4i:
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
-
16N.2.hl.TZ0.4j:
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
-
16N.2.hl.TZ0.4k:
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
17M.1.hl.TZ1.30:
Which statement is correct for the overall reaction in a voltaic cell?
2AgNO3(aq) + Ni(s) → 2Ag(s) + Ni(NO3)2(aq) E θ= +1.06 V
A. Electrons flow from Ag electrode to Ni electrode.
B. Ni is oxidized to Ni2+ at the cathode (negative electrode).
C. Ag+ is reduced to Ag at the anode (positive electrode).
D. Ag has a more positive standard electrode potential value than Ni.
-
17M.1.hl.TZ1.31:
In the electrolysis of aqueous potassium nitrate, KNO3(aq), using inert electrodes, 0.1 mol of a gas was formed at the cathode (negative electrode).
Which is correct?
-
17M.2.hl.TZ1.3b.i:
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
-
17M.2.hl.TZ1.3b.ii:
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
-
17M.2.hl.TZ1.3c.ii:
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
- 17M.1.hl.TZ2.30: What is the standard half-cell potential of copper if the “zero potential reference electrode”...
- 17M.1.hl.TZ2.31: What are the relative volumes of gas given off at E and F during electrolysis of the two cells...
-
17M.2.hl.TZ2.2b.i:
Corrosion of iron is similar to the processes that occur in a voltaic cell. The initial steps involve the following half-equations:
Fe2+(aq) + 2e– Fe(s)
O2(g) + H2O(l) + 2e– 2OH–(aq)
Calculate E θ, in V, for the spontaneous reaction using section 24 of the data booklet.
-
17M.2.hl.TZ2.2b.ii:
Calculate the Gibbs free energy, ΔG θ, in kJ, which is released by the corrosion of 1 mole of iron. Use section 1 of the data booklet.
-
17M.2.hl.TZ2.2c:
Zinc is used to galvanize iron pipes, forming a protective coating. Outline how this process prevents corrosion of the iron pipes.
-
17N.1.hl.TZ0.31:
What are the products when an aqueous solution of copper(II) sulfate is electrolysed using inert graphite electrodes?
-
17N.2.hl.TZ0.7b:
Predict, giving a reason, the direction of movement of electrons when the standard nickel and manganese half-cells are connected.
-
17N.2.hl.TZ0.7c:
Calculate the cell potential, in V, when the standard iodine and manganese half-cells are connected.
-
17N.2.hl.TZ0.7e:
State and explain the products of electrolysis of a concentrated aqueous solution of sodium chloride using inert electrodes. Your answer should include half-equations for the reaction at each electrode.
- 18M.1.hl.TZ1.30: Which combination would electroplate an object with copper?
- 18M.1.hl.TZ1.31: What does not affect the mass of products formed in electrolysis of an aqueous solution? A. ...
-
18M.2.hl.TZ1.6d:
Calculate the cell potential, in V, using section 24 of the data booklet.
-
18M.2.hl.TZ1.6e:
Determine the loss in mass of one electrode if the mass of the other electrode increases by 0.10 g.
-
18M.1.hl.TZ2.30:
Two cells undergoing electrolysis are connected in series.

If g of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
Ar(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
A.
B.
C.
D.
-
18M.1.hl.TZ2.31:
What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?

-
18M.2.hl.TZ2.3c.v:
Deduce the gas formed at the anode (positive electrode) when graphite is used in place of copper.
-
18M.2.hl.TZ2.4b:
The change in the free energy for the reaction under standard conditions, ΔGΘ, is −514 kJ at 298 K.
Determine the value of EΘ, in V, for the reaction using sections 1 and 2 of the data booklet.
-
18M.2.hl.TZ2.4c:
Calculate the standard electrode potential, in V, for the BrO3−/Br− reduction half‑equation using section 24 of the data booklet.
- 18M.1.hl.TZ1.29: What are the products of electrolysis when concentrated calcium bromide solution is electrolysed...
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.1.hl.TZ0.31:
Consider the standard electrode potentials:
Cr3+ (aq) + 3e− Cr (s) EΘ = −0.74 V
Hg2+ (aq) + 2e− Hg (l) EΘ = +0.85 V
What is the cell potential, in V, for the voltaic cell?
2Cr (s) + 3Hg2+ (aq) → 3Hg (l) + 2Cr3+ (aq)
A. −1.59
B. +0.11
C. +1.07
D. +1.59
-
18N.2.hl.TZ0.1d:
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
-
18N.2.hl.TZ0.3d.iii:
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
-
19M.2.hl.TZ1.6f(i):
Calculate the standard electrode potential, in V, when the Fe2+ (aq) | Fe (s) and Cu2+ (aq) | Cu (s) standard half-cells are connected at 298 K. Use section 24 of the data booklet.
-
19M.2.hl.TZ1.6f(ii):
Calculate ΔGθ, in kJ, for the spontaneous reaction in (f)(i), using sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ1.7:
An aqueous solution of silver nitrate, AgNO3 (aq), can be electrolysed using platinum electrodes.
Formulate the half-equations for the reaction at each electrode during electrolysis.
Cathode (negative electrode):
Anode (positive electrode):
-
19M.2.hl.TZ2.4b(ii):
A scientist wants to investigate the catalytic properties of a thin layer of rhenium metal on a graphite surface.
Describe an electrochemical process to produce a layer of rhenium on graphite.
-
19M.2.hl.TZ2.4e(iii):
Predict, giving a reason, whether the reduction of ReO4− to [Re(OH)2]2+ would oxidize Fe2+ to Fe3+ in aqueous solution. Use section 24 of the data booklet.
- 19M.1.hl.TZ1.30: Which factors affect the amount of product formed at the cathode during electrolysis of molten...
-
19M.1.hl.TZ1.31:
Which is not a requirement of the standard hydrogen electrode (SHE)?
A. V = 1 dm3
B. p(H2) = 100 kPa
C. use of platinum as the electrode material
D. [H3O+] = 1 mol dm−3
-
19M.1.hl.TZ2.30:
Consider the following table of standard electrode potentials.
Which is the strongest oxidizing agent?
A. Pb2+
B. Pb
C. Al3+
D. Al
-
19M.1.hl.TZ2.31:
What are the products when concentrated KBr (aq) is electrolyzed?
-
19N.2.hl.TZ0.6c(iii):
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
-
19N.2.hl.TZ0.6c(iv):
Deduce the half-equation for the formation of the gas identified in (c)(iii).
- 19N.2.hl.TZ0.6e(i): Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24...
- 19N.2.hl.TZ0.6e(ii): Comment on the spontaneity of the disproportionation reaction at 298 K.
-
19N.2.hl.TZ0.6e(iii):
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
-
19N.2.hl.TZ0.6e(iv):
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
-
19N.1.hl.TZ0.32:
Three cells with platinum electrodes are connected in series to a DC power supply.
What is the ratio of moles formed at each cathode (negative electrode)?
- 20N.1.hl.TZ0.30: Which conditions deposit the greatest mass of copper when solutions containing copper ions are...
-
20N.1.hl.TZ0.31:
Which statement is correct when a zinc spoon is electroplated with silver?
A. The cathode (negative electrode) is made of silver.
B. The anode (positive electrode) is the zinc spoon.
C. The anode (positive electrode) is made of silver.
D. The electrolyte is zinc sulfate solution.
-
20N.2.hl.TZ0.4d(ii):
Calculate the standard cell potential, in , for the cell at . Use section 24 of the data booklet
-
20N.2.hl.TZ0.4d(iii):
Calculate the standard free energy change, , in , for the cell using sections 1 and 2 of the data booklet.
-
20N.2.hl.TZ0.6c:
The electron configuration of copper makes it a useful metal.
Copper plating can be used to improve the conductivity of an object.
State, giving your reason, at which electrode the object being electroplated should be placed.
-
20N.3.hl.TZ0.13a:
Write the balanced equation for the reaction in this voltaic cell.
-
20N.3.hl.TZ0.13b:
Calculate the cell potential for and at . Use sections 1, 2 and 24 of the data booklet.
-
20N.3.hl.TZ0.13c:
Predict, giving a reason, how an increase in temperature affects the potential of this cell.
- 21M.1.hl.TZ1.30: Which gives the equation and cell potential of the spontaneous reaction?
-
21M.1.hl.TZ1.31:
What are the products when concentrated aqueous copper (II) chloride is electrolysed using platinum electrodes?
-
21M.1.hl.TZ2.30:
What would be the electrode potential, E⦵, of the Mn2+ (aq)|Mn (s) half-cell if Fe3+ (aq)|Fe2+ (aq) is used as the reference standard?
Mn2+ (aq) + 2e− Mn (s) E⦵ = −1.18 V
Fe3+ (aq) + e− Fe2+ (aq) E⦵ = +0.77 VA. −1.95 V
B. −0.41 V
C. +0.41 V
D. +1.95 V
-
21M.1.hl.TZ2.31:
What happens to the mass of each copper electrode when aqueous copper(II) sulfate solution is electrolysed?
-
21M.2.hl.TZ1.3d:
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
- 21M.2.hl.TZ1.3e: The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure...
-
21M.2.hl.TZ2.3c:
Calculate the cell potential using section 24 of the data booklet.
-
21M.2.hl.TZ2.3d:
Calculate the Gibbs free energy change, ΔG⦵, in kJ, for the cell, using section 1 of the data booklet.
-
21N.1.hl.TZ0.30:
Consider the following standard electrode potentials:
Which species will react with each other spontaneously under standard conditions?
A. Zn2+ (aq) + Pb (s)B. Pb2+ (aq) + Br2 (l)
C. Zn (s) + Br− (aq)
D. Pb (s) + Br2 (l)
-
21N.1.hl.TZ0.31:
Which aqueous solutions produce oxygen gas during electrolysis?
I. Dilute CuCl2 (aq) with inert electrodes
II. Dilute FeSO4 (aq) with inert electrodes
III. Dilute CuCl2 (aq) with copper electrodesThe standard electrode potentials are provided in the table:
A. I and II onlyB. I and III only
C. II and III only
D. I, II and III
-
21N.2.hl.TZ0.8:
The standard electrode potential of zinc can be measured using a standard hydrogen electrode (SHE).
Draw and annotate the diagram to show the complete apparatus required to measure the standard electrode potential of zinc.
-
22M.1.hl.TZ1.30:
What are the products when dilute aqueous copper (II) nitrate is electrolysed using platinum electrodes?
E⦵ (Cu | Cu2+) = –0.34 V.
-
22M.1.hl.TZ1.31:
In the electrolysis apparatus shown, 0.59 g of Ni is deposited on the cathode of the first cell.
What is the mass of Ag deposited on the cathode of the second cell?
A. 0.54 gB. 0.59 g
C. 1.08 g
D. 2.16 g
-
22M.1.hl.TZ2.30:
Which E⦵ value, in V, for the reaction Mn (s) + Zn2+ (aq) → Mn2+ (aq) + Zn (s) can be deduced from the following equations?
Mn (s) + 2Ag+ (aq) → Mn2+ (aq) + 2Ag (s) E⦵ = 1.98 V
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) E⦵ = 1.10 V
Cu (s) + 2Ag+ (aq) → Cu2+ (aq) + 2Ag (s) E⦵ = 0.46 V
A. 0.42
B. 1.34
C. 2.62
D. 3.54
- 22M.1.hl.TZ2.31: What is the order of increasing mass deposited by this electrolytic cell? Ar Ag = 108, Cu =...
-
22M.2.hl.TZ1.2b(i):
Calculate the standard potential, in V, of a cell formed by magnesium and steel half-cells. Use section 24 of the data booklet and assume steel has the standard electrode potential of iron.
-
22M.2.hl.TZ1.2b(ii):
Calculate the free energy change, ΔG⦵, in kJ, of the cell reaction. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ1.2b(iii):
This cell causes the electrolytic reduction of water on the steel. State the half-equation for this reduction.
-
22M.2.hl.TZ2.3a(i):
Iron(II) is oxidized by bromine.
2Fe2+ (aq) + Br2 (l) 2Fe3+ (aq) + 2Br− (aq)
Calculate the E⦵cell, in V, for the reaction using section 24 of the data booklet.
-
22M.2.hl.TZ2.3a(ii):
Determine, giving a reason, if iodine will also oxidize iron(II).
Topic 20: Organic chemistry
- 16N.1.hl.TZ0.36: Which is correct for the conversion of propanal to propyl methanoate?
- 16N.1.hl.TZ0.37: Which statement is correct for a pair of enantiomers under the same conditions? A. A racemic...
-
16N.2.hl.TZ0.5d:
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
- 16N.2.hl.TZ0.6a: Draw the three-dimensional shape of each enantiomer of this isomer showing their spatial...
- 16N.2.hl.TZ0.6b: When one enantiomer undergoes substitution by alkaline hydrolysis approximately 75 % of the...
- 16N.2.hl.TZ0.6c: Suggest why the rate of alkaline hydrolysis of an enantiomer of iodopropane is greater than that...
- 17M.1.hl.TZ1.36: What is the product of the reduction of 2-methylbutanal? A. 2-methylbutan-1-ol B. ...
- 17M.1.hl.TZ1.37: Which molecule is chiral? A. 2-chlorobutane B. 2,2-dichloropentane C. ...
-
17M.2.hl.TZ1.6c.iii:
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
-
17M.2.hl.TZ1.7c:
State the reagents used to convert benzene to nitrobenzene and the formula of the electrophile formed.
- 17M.2.hl.TZ1.7d: Explain the mechanism for the nitration of benzene, using curly arrows to show the movement of...
-
17M.2.hl.TZ1.7e:
State the reagents used in the two-stage conversion of nitrobenzene to aniline.
-
17M.3.hl.TZ1.17:
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
-
17M.3.hl.TZ1.28a:
Describe what happens to plane-polarized light when it passes through a solution of an optically active compound.
-
17M.3.hl.TZ1.28b:
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
- 17M.1.hl.TZ2.35: Which pair of isomers always shows optical activity? A. Cis-trans B. Enantiomers C. ...
-
17M.1.hl.TZ2.37:
In which order should the reagents be used to convert benzene into phenylamine (aniline)?
-
17M.2.hl.TZ2.7c.i:
State the reagents and the name of the mechanism for the nitration of benzene.
-
17M.2.hl.TZ2.7d:
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
- 17N.1.hl.TZ0.33: Propene reacts separately with H2O/H+ and H2/Ni to give products X and Z respectively. What...
- 17N.1.hl.TZ0.35: What is the product of the reaction between pentan-2-one and sodium borohydride, NaBH4? A....
- 17N.1.hl.TZ0.37: What is the number of optical isomers of isoleucine? A. 0 B. 2 C. 4 D. 8
- 17N.2.hl.TZ0.8a.iv: Deduce, giving a reason, which of the two compounds can show optical activity.
- 17N.2.hl.TZ0.8a.v: Draw three-dimensional representations of the two enantiomers.
-
17N.2.hl.TZ0.8c:
State the reagents used in the nitration of benzene.
-
17N.2.hl.TZ0.8d:
State an equation for the formation of NO2+.
-
17N.2.hl.TZ0.8e:
Explain the mechanism of the reaction between 2-bromo-2-methylpropane, (CH3)3CBr, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
- 18M.1.hl.TZ1.35: What is name of this compound applying IUPAC rules? A. E 1-bromo-1-chlorobut-1-ene B. ...
-
18M.1.hl.TZ1.36:
Which molecule contains a chiral carbon?
A. CH3CH2CHBrCH2CH3
B. CH3CH2CHBrCH3
C. CH2BrCH(CH3)CH2Br
D. CH3CH2CH2CH2CH2Br
- 18M.1.hl.TZ1.37: Which reagents are needed to convert nitrobenzene to phenylamine in 2 steps?
-
18M.2.hl.TZ1.7a:
Compare and contrast the mechanisms by which 1-chlorobutane, CH3CH2CH2CH2Cl, and 2-chloro-2-methylpropane, (CH3)3CCl, react with aqueous sodium hydroxide, giving two similarities and one difference.
-
18M.2.hl.TZ1.7b:
Outline why the rate of reaction of the similar bromo-compounds is faster.
-
18M.2.hl.TZ1.7c.ii:
Suggest how this product could be synthesized in one step from butanoic acid.
- 18M.1.hl.TZ2.35: Which is the correct combination of substitution reaction mechanisms?
-
18M.1.hl.TZ2.36:
Propene is reacted first with hydrogen chloride to produce X which is then reacted with aqueous sodium hydroxide to give Y. Finally, Y is reacted with excess acidified potassium dichromate solution.
What is the major product, Z?
A. CH3CH(OH)CH3
B. CH3COCH3
C. CH3CH2CHO
D. CH3(CH2)2COOH
- 18M.1.hl.TZ2.37: Which isomers exist as non-superimposable mirror images? A. cis-trans isomers B. ...
-
18M.2.hl.TZ2.9b.i:
State the type of bond fission that takes place in a SN1 reaction.
-
18M.2.hl.TZ2.9b.ii:
State the type of solvent most suitable for the reaction.
-
18M.2.hl.TZ2.9b.iii:
Draw the structure of the intermediate formed stating its shape.
-
18M.2.hl.TZ2.9b.iv:
Suggest, giving a reason, the percentage of each isomer from the SN1 reaction.
-
18M.2.hl.TZ2.9c:
Nitrobenzene, C6H5NO2, can be converted to phenylamine via a two-stage reaction.
In the first stage, nitrobenzene is reduced with tin in an acidic solution to form an intermediate ion and tin(II) ions. In the second stage, the intermediate ion is converted to phenylamine in the presence of hydroxide ions.
Formulate the equation for each stage of the reaction.
-
18M.3.hl.TZ2.8e:
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
- 18N.1.hl.TZ0.35: Which statement about the reaction of a hydroxide ion with the organic reagent is...
- 18N.1.hl.TZ0.36: What is the major product of the reaction of HBr with but-1-ene? A. 1-bromobutane B. ...
-
18N.1.hl.TZ0.37:
How many chiral carbon atoms are present in one molecule of (CH3)2CHCHClCHBrCH3?
A. 0
B. 1
C. 2
D. 3
- 18N.2.hl.TZ0.6e.i: State a suitable reagent for the reduction of butanoic acid.
-
18N.2.hl.TZ0.6e.ii:
Deduce the product of the complete reduction reaction in (e)(i).
- 18N.2.hl.TZ0.8b.ii: State, giving a reason, whether methyloxirane can form cis-trans isomers.
- 18N.2.hl.TZ0.9c: Explain the mechanism of the reaction between 1-bromopropane with aqueous sodium hydroxide using...
-
19M.2.hl.TZ1.1c(i):
Write the equation for the production of the active nitrating agent from concentrated sulfuric and nitric acids.
-
19M.2.hl.TZ1.1c(ii):
Explain the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
-
19M.2.hl.TZ1.1e:
The organic product is not optically active. Discuss whether or not the organic product is a racemic mixture.
-
19M.2.hl.TZ1.2i:
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
-
19M.2.hl.TZ2.6c:
The benzene ring of phenylethene reacts with the nitronium ion, NO2+, and the C=C double bond reacts with hydrogen bromide, HBr.
Compare and contrast these two reactions in terms of their reaction mechanisms.
Similarity:
Difference:
-
19M.2.hl.TZ2.6d(i):
Outline why the major product, C6H5–CHBr–CH3, can exist in two forms and state the relationship between these forms.
Two forms:
Relationship:
-
19M.2.hl.TZ2.6d(ii):
The minor product, C6H5–CH2–CH2Br, can exist in different conformational forms (isomers).
Outline what this means.
-
19M.2.hl.TZ2.6e:
The minor product, C6H5–CH2–CH2Br, can be directly converted to an intermediate compound, X, which can then be directly converted to the acid C6H5–CH2–COOH.
C6H5–CH2–CH2Br → X → C6H5–CH2–COOH
Identify X.
-
19M.3.hl.TZ1.5a:
Label with an asterisk, *, the chiral carbon atom.
-
19M.1.hl.TZ1.33:
Which is a major product of the electrophilic addition of hydrogen chloride to propene?
A. ClCH2CH=CH2
B. CH3CH(Cl)CH3
C. CH3CH2CH2Cl
D. CH3CH=CHCl
- 19M.1.hl.TZ1.35: Which solvent is aprotic? A. H2O B. C6H5CH3 C. CH3OH D. CH3NH2
- 19M.1.hl.TZ1.36: Which statement is not correct regarding benzene? A. It is planar. B. The ring contains...
- 19M.1.hl.TZ1.37: Which compound can exist as cis- and trans-isomers?
- 19M.1.hl.TZ2.36: Which compound exists as two configurational isomers? A. CBr2=CH2 B. CH2=CHBr C....
-
19M.1.hl.TZ2.37:
Which class of compound is formed when a ketone is reduced?
A. primary alcohol
B. secondary alcohol
C. ether
D. carboxylic acid
- 19N.2.hl.TZ0.3a(iii): Outline why it is the major product.
-
19N.2.hl.TZ0.3a(iv):
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
- 19N.2.hl.TZ0.3b(ii): Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium...
-
19N.2.hl.TZ0.3b(iii):
Sketch the mechanism using curly arrows to represent the movement of electrons.
- 19N.3.hl.TZ0.26a: State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data...
-
19N.1.hl.TZ0.36:
In which compound is the halogen substituted the most rapidly by aqueous hydroxide ions?
A. (CH3)3CCl
B. (CH3)3CI
C. CH3CH2CH2CH2Cl
D. CH3CH2CH2CH2I
- 19N.1.hl.TZ0.37: Which can be reduced to an aldehyde? A. Butanone B. Butan-1-ol C. Butanoic acid D. Butan-2-ol
-
19N.1.hl.TZ0.38:
Which can show optical activity?
A. CHBrCHCl
B. CH3CH2CHBrCH2CH3
C. (CH3)2CBrCl
D. CH3CH2CH(CH3)Br
-
20N.1.hl.TZ0.35:
Which is the electrophile in the nitration of benzene?
A.
B.
C.
D.
-
20N.1.hl.TZ0.36:
What will be the major product in the reaction between but-1-ene and ?
A. 2-bromobut-1-ene
B. 1-bromobut-1-ene
C. 2-bromobutane
D. 1-bromobutane
-
20N.1.hl.TZ0.37:
Which molecule has an enantiomer?
A.
B.
C.
D.
-
20N.2.hl.TZ0.1d(iii):
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
-
20N.2.hl.TZ0.2g(i):
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
-
20N.2.hl.TZ0.2g(ii):
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
-
21M.1.hl.TZ1.35:
Which is most likely to hydrolyse via a SN1 mechanism?
A. CH3CHBrCH2CH3
B. (CH3)2CHBr
C. (CH3)3CBr
D. CH3CH2CH2CH2Br
- 21M.1.hl.TZ1.36: What is the product of the reaction of benzene with a mixture of concentrated nitric and sulfuric...
- 21M.1.hl.TZ1.37: How many chiral centres are there in the following molecule? A. 2 B. 3 C. 4 D. 6
- 21M.1.hl.TZ2.35: Which compound shows cis-trans isomerism? A. CH3CH=CCl2 B. CCl2=CH2 C. D.
-
21M.1.hl.TZ2.36:
Which compound rotates the plane of plane-polarized light?
A. CH3C(CH3)ClCH3
B. CH3CH2CHClCH3
C. CH3C(Cl)2CH3
D. CH3CClBrCH3
-
21M.2.hl.TZ1.5e(i):
Sketch the mechanism for the reaction of propene with hydrogen bromide using curly arrows.
-
21M.2.hl.TZ1.5e(ii):
Explain why the major organic product is 2-bromopropane and not 1-bromopropane.
- 21M.2.hl.TZ2.4e: Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly...
- 21M.2.hl.TZ2.4f: Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
- 21M.2.hl.TZ2.4h(i): Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
- 21M.2.hl.TZ2.4h(ii): Outline how two enantiomers can be distinguished using a polarimeter.
-
21M.2.hl.TZ1.5e(ii):
Explain why the major organic product is 2-bromopropane and not 1-bromopropane.
- 21N.1.hl.TZ0.35: Which statement is correct about configurational isomers? A. Configurational isomers can only...
-
21N.1.hl.TZ0.36:
Which product is formed when CH3COCH2CH3 is reduced with sodium borohydride?
A. CH3CH2CH2CHOB. CH3CH2CH2CH2OH
C. CH3CH(OH)CH2CH3
D. CH3CH2CH2COOH
- 21N.1.hl.TZ0.37: Which attacking species is matched with its mechanism of reaction?
- 21N.2.hl.TZ0.10b(i): State, giving a reason, if but-1-ene exhibits cis-trans isomerism.
- 21N.2.hl.TZ0.10b(iii): Explain the mechanism of the reaction between but-1-ene with hydrogen iodide, using curly arrows...
- 21N.2.hl.TZ0.10b(iv): State, giving a reason, if the product of this reaction exhibits stereoisomerism.
- 21N.2.hl.TZ0.10e: Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
- 22M.1.hl.TZ1.35: What are the type of reaction and role of the nitronium ion, NO2+, in the following...
- 22M.1.hl.TZ1.36: What is molecule Z that is formed in step 1 of this synthetic route?
- 22M.1.hl.TZ1.37: What are the E/Z designations of these stereoisomers?
- 22M.1.hl.TZ2.32: Which sequence of reagents converts propene to propanone?
- 22M.1.hl.TZ2.37: What is the product of the reaction of propanal with lithium aluminium hydride, LiAlH4? A. ...
-
22M.2.hl.TZ1.5a(v):
Identify the isomer of Compound B that exists as optical isomers (enantiomers).
-
22M.2.hl.TZ1.5b(ii):
Explain why the reaction produces more (CH3)3COH than (CH3)2CHCH2OH.
-
22M.2.hl.TZ1.5d(iii):
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
-
22M.2.hl.TZ1.6b(i):
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
- 22M.2.hl.TZ1.6b(ii): Draw the structural formula of the carbocation intermediate produced when this electrophile...
-
22M.2.hl.TZ2.8d(i):
Draw the full structural formula of (Z)-but-2-ene.
-
22M.2.hl.TZ2.8d(v):
Predict, giving a reason, the major product of reaction between but-1-ene and steam.
-
22M.2.hl.TZ2.8e(i):
Explain the mechanism of the reaction between 1-bromopropane, CH3CH2CH2Br, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Topic 21: Measurement and analysis
-
16N.1.hl.TZ0.40:
Which property explains why tetramethylsilane, Si(CH3)4, can be used as a reference standard in 1H NMR spectroscopy?
A. It has a high boiling point.
B. It is a reactive compound.
C. All its protons are in the same chemical environment.
D. It gives multiple signals.
- 16N.2.hl.TZ0.1f: Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
-
17M.1.hl.TZ1.40:
Which technique is used to determine the bond lengths and bond angles of a molecule?
A. X-ray crystallography
B. Infrared (IR) spectroscopy
C. Mass spectroscopy
D. 1H NMR spectroscopy
-
17M.2.hl.TZ1.6b.ii:
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
-
17M.2.hl.TZ1.6b.iii:
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
- 17M.1.hl.TZ2.40: Which technique can be used to identify bond length and bond angle? A. 1H NMR...
-
17M.2.hl.TZ2.7a.i:
Deduce what information can be obtained from the 1H NMR spectrum.
-
17M.2.hl.TZ2.7a.iii:
Suggest the structural formula of this compound.
-
17M.3.hl.TZ2.21c.ii:
Predict the chemical shift and the splitting pattern seen for the hydrogens on the carbon atom circled in the diagram. Use section 27 of the data booklet.
- 17N.1.hl.TZ0.39: Which compound gives this 1H NMR spectrum? A. CH3CH2OCH2CH3 B. CH3CH2OH C. CH3CH2CH3 D....
- 18M.1.hl.TZ1.40: Which would be the most effective method to distinguish between liquid propan-1-ol and...
-
18M.2.hl.TZ1.1l.ii:
Predict the splitting pattern of the 1H NMR spectrum of urea.
-
18M.2.hl.TZ1.1l.iii:
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
-
18M.2.hl.TZ2.9a.ii:
Mass spectra A and B of the two isomers are given.

Explain which spectrum is produced by each compound using section 28 of the data booklet.
-
18M.3.hl.TZ2.27b:
Predict the chemical shifts and integration for each signal in the 1H NMR spectrum for ethanol using section 27 of the data booklet.
- 18N.1.hl.TZ0.40: Which technique may be used to find the bond lengths and bond angles within a molecule? A. ...
- 18N.2.hl.TZ0.8c: Predict the chemical shift and splitting pattern of the signal produced by the hydrogen atoms...
-
19M.2.hl.TZ1.2a:
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
-
19M.2.hl.TZ1.2b:
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
-
19M.2.hl.TZ2.1c(v):
Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
-
19M.1.hl.TZ1.40:
Which can be identified using infrared (IR) spectroscopy?
A. functional groups
B. molar mass
C. 3-D configuration
D. bond angle
-
19N.3.hl.TZ0.7:
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.
Using X-rays of wavelength 1.54 × 10−10 m, the first bright spots were produced at an angle θ of 22.3° from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
-
19N.1.hl.TZ0.40:
Which is the 1H NMR spectrum of tetramethylsilane, TMS, (CH3)4Si?
-
20N.1.hl.TZ0.40:
Which compound with the molecular formula has this high resolution ?
From: libretexts.org. Courtesy of Chris Schaller, Professor (Chemistry)
at College of Saint Benedict/Saint John’s University.A. but-3-en-2-ol,
B. butanal,
C. butanone,
D. but-3-en-1-ol,
-
20N.2.hl.TZ0.1d(v):
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
-
21M.1.hl.TZ1.40:
Which compound produces the following 1H NMR spectrum?
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
A. PropaneB. Propanone
C. Propanal
D. 2,2-dimethylpropane
-
21M.1.hl.TZ2.40:
What information can be deduced from the splitting pattern of 1H NMR signals?
A. total number of hydrogen atoms in a compound
B. number of hydrogen atoms on adjacent atom(s)
C. functional group on which hydrogen atoms are located
D. number of hydrogen atoms in a particular chemical environment
- 21M.2.hl.TZ1.1d(ii): State a technique that could be used to determine the crystal structure of the solid compound.
- 21M.2.hl.TZ1.5b(ii): Deduce the chemical shift of this signal. Use section 27 of the data booklet.
- 21N.1.hl.TZ0.40: Which substance has the following 1H NMR spectrum? SDBS, National Institute of Advanced...
-
21N.2.hl.TZ0.1d:
Predict the number of 1H NMR signals, and splitting pattern of the –CH3 seen for propanone (CH3COCH3) and propanal (CH3CH2CHO).
-
22M.1.hl.TZ1.38:
Which compound produces the following 1H NMR spectrum?
[Spectral Database for Organic Compounds, SDBS. SDBS Compounds and Spectral Search. [graph] Available at:
https://sdbs.db.aist.go.jp [Accessed 3 January 2019].]
A. propanalB. propanone
C. propane
D. methlypropane
- 22M.2.hl.TZ1.6a(iv): State a technique used to determine the length of the bonds between N and O in solid HNO3.
-
22M.2.hl.TZ1.6b(iii):
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
-
22M.2.hl.TZ2.8e(ii):
Deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane.
