| Date | May 2021 | Marks available | 2 | Reference code | 21M.2.hl.TZ1.1 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Sketch | Question number | 1 | Adapted from | N/A |
Question
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
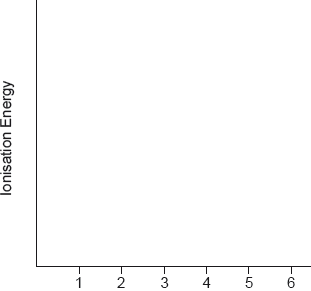
Sketch the first eight successive ionisation energies of sulfur.
Describe the bonding in this type of solid.
State a technique that could be used to determine the crystal structure of the solid compound.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Markscheme
mobile/delocalized «sea of» electrons
Any two of:
forms acidic oxides «rather than basic oxides» ✔
forms covalent/bonds compounds «with other non-metals» ✔
forms anions «rather than cations» ✔
behaves as an oxidizing agent «rather than a reducing agent» ✔
Award [1 max] for 2 correct non-chemical properties such as non-conductor, high ionisation energy, high electronegativity, low electron affinity if no marks for chemical properties are awarded.
two regions of small increases AND a large increase between them✔
large increase from 6th to 7th ✔
Accept line/curve showing these trends.
electrostatic attraction ✔
between oppositely charged ions/between Fe2+ and S2− ✔
X-ray crystallography ✔
1s2 2s2 2p6 3s2 3p6 ✔
Do not accept “[Ne] 3s2 3p6”.
«valence» electrons further from nucleus/extra electron shell/ electrons in third/3s/3p level «not second/2s/2p»✔
Accept 2,8 (for O2–) and 2,8,8 (for S2–)
allows them to explain the properties of different compounds/substances
OR
enables them to generalise about substances
OR
enables them to make predictions ✔
Accept other valid answers.
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g) ✔
Accept any correct ratio.
+6
OR
−2 to +4 ✔
Accept “6/VI”.
Accept “−II, 4//IV”.
Do not accept 2- to 4+.
sulfur dioxide/SO2 causes acid rain ✔
Accept sulfur dioxide/SO2/dust causes respiratory problems
Do not accept just “causes respiratory problems” or “causes acid rain”.
disrupts the regular arrangement «of iron atoms/ions»
OR
carbon different size «to iron atoms/ions» ✔
prevents layers/atoms sliding over each other ✔