| Date | May 2021 | Marks available | 3 | Reference code | 21M.2.hl.TZ1.8 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Sketch | Question number | 8 | Adapted from | N/A |
Question
Propanoic acid, CH3CH2COOH, is a weak organic acid.
Calculate the pH of 0.00100 mol dm–3 propanoic acid solution. Use section 21 of the data booklet.
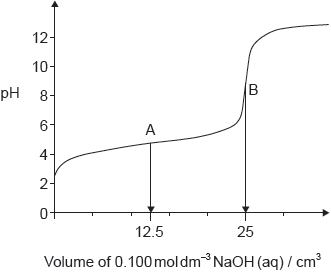
Sketch the general shape of the variation of pH when 50 cm3 of 0.001 mol dm–3 NaOH (aq) is gradually added to 25 cm3 of 0.001 mol dm–3 CH3CH2COOH (aq).
Markscheme
Ka = 10−4.87 / 1.35 × 10−5 ✔
[H+] = «» 1.16 × 10−4 «mol dm−3» ✔
pH = 3.94 ✔
Accept alternative methods of calculation.
Award [3] for correct final answer.
Award [3] for 3.96 {answer if solved by quadratic}.
Any three of:
correct “S” shape ✔
equivalence point at 25 cm3 ✔
final pH tends to 11 ✔
pH at equivalence point >7 ✔
starting pH between 3.8 - 4 ✔
pH at half equivalence approx. 5 ✔
Do not penalize for incorrect points.
Award any 3 correct.