| Date | November 2020 | Marks available | 3 | Reference code | 20N.3.hl.TZ0.13 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Calculate | Question number | 13 | Adapted from | N/A |
Question
A voltaic cell is made up of nickel and magnesium half-cells.
Write the balanced equation for the reaction in this voltaic cell.
Calculate the cell potential for and at . Use sections 1, 2 and 24 of the data booklet.
Predict, giving a reason, how an increase in temperature affects the potential of this cell.
Markscheme
✔
Accept a balanced molecular equation such as “”.
✔
AND ✔
✔
Award [3] for correct final answer.
cell potential/ increases AND increasing temperature favours forward reaction
OR
cell potential/ increases AND becomes more negative
OR
cell potential/ increases AND becomes more negative ✔
Accept any correct mathematical explanation using the Nernst equation.
Examiners report
Nearly all were able to write the balanced equation for the reaction occurring in the voltaic cell.
The cell potential numerical problem was well executed and many scored all three marks, by calculating the final answer as +2.17 V. The most common errors related to either incorrectly calculating Q or identifying n = 2 in the intermediate, numerical step.
This was often gained as an easy subsequent mark for those that scored full marks in (b). The majority approached this problem by applying a mathematical explanation using the Nernst equation.
Syllabus sections
-
22M.2.hl.TZ1.2b(i):
Calculate the standard potential, in V, of a cell formed by magnesium and steel half-cells. Use section 24 of the data booklet and assume steel has the standard electrode potential of iron.
-
22M.2.hl.TZ1.2b(iii):
This cell causes the electrolytic reduction of water on the steel. State the half-equation for this reduction.
-
22M.1.hl.TZ1.30:
What are the products when dilute aqueous copper (II) nitrate is electrolysed using platinum electrodes?
E⦵ (Cu | Cu2+) = –0.34 V.
-
18M.1.hl.TZ2.31:
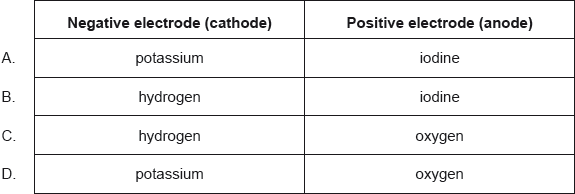
What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?
- 22M.1.hl.TZ2.31: What is the order of increasing mass deposited by this electrolytic cell? Ar Ag = 108, Cu...
- 16N.1.hl.TZ0.32: Which signs for both Eθcell and ΔGθ result in a spontaneous redox reaction occurring under...
- 18M.1.hl.TZ1.29: What are the products of electrolysis when concentrated calcium bromide solution is...
- 18M.1.hl.TZ1.30: Which combination would electroplate an object with copper?
- 18M.1.hl.TZ1.31: What does not affect the mass of products formed in electrolysis of an aqueous solution? A. ...
-
18M.2.hl.TZ2.3c.v:
Deduce the gas formed at the anode (positive electrode) when graphite is used in place of copper.
-
17M.2.hl.TZ2.2c:
Zinc is used to galvanize iron pipes, forming a protective coating. Outline how this process prevents corrosion of the iron pipes.
-
18M.2.hl.TZ1.6d:
Calculate the cell potential, in V, using section 24 of the data booklet.
- 17M.1.hl.TZ2.31: What are the relative volumes of gas given off at E and F during electrolysis of the two...
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is...
-
17M.1.hl.TZ1.31:
In the electrolysis of aqueous potassium nitrate, KNO3(aq), using inert electrodes, 0.1 mol of a gas was formed at the cathode (negative electrode).
Which is correct?
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
16N.2.hl.TZ0.4j:
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
- 19M.1.hl.TZ1.30: Which factors affect the amount of product formed at the cathode during electrolysis of...
-
19M.1.hl.TZ2.31:
What are the products when concentrated KBr (aq) is electrolyzed?
-
17N.2.hl.TZ0.7b:
Predict, giving a reason, the direction of movement of electrons when the standard nickel and manganese half-cells are connected.
-
19M.1.hl.TZ1.31:
Which is not a requirement of the standard hydrogen electrode (SHE)?
A. V = 1 dm3
B. p(H2) = 100 kPa
C. use of platinum as the electrode material
D. [H3O+] = 1 mol dm−3
-
18N.2.hl.TZ0.3d.iii:
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
-
18N.2.hl.TZ0.1d:
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
-
22M.2.hl.TZ2.3a(i):
Iron(II) is oxidized by bromine.
2Fe2+ (aq) + Br2 (l) 2Fe3+ (aq) + 2Br− (aq)
Calculate the E⦵cell, in V, for the reaction using section 24 of the data booklet.
-
19M.1.hl.TZ2.30:
Consider the following table of standard electrode potentials.
Which is the strongest oxidizing agent?
A. Pb2+
B. Pb
C. Al3+
D. Al
-
17N.1.hl.TZ0.31:
What are the products when an aqueous solution of copper(II) sulfate is electrolysed using inert graphite electrodes?
- 17M.1.hl.TZ2.30: What is the standard half-cell potential of copper if the “zero potential reference...
-
16N.1.hl.TZ0.33:
An iron rod is electroplated with silver. Which is a correct condition for this process?
A. The silver electrode is the positive electrode.
B. The iron rod is the positive electrode.
C. The electrolyte is iron(II) sulfate.
D. Oxidation occurs at the negative electrode.
-
17M.2.hl.TZ1.3b.i:
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
-
17N.2.hl.TZ0.7c:
Calculate the cell potential, in V, when the standard iodine and manganese half-cells are connected.
-
21N.1.hl.TZ0.30:
Consider the following standard electrode potentials:
Which species will react with each other spontaneously under standard conditions?
A. Zn2+ (aq) + Pb (s)B. Pb2+ (aq) + Br2 (l)
C. Zn (s) + Br− (aq)
D. Pb (s) + Br2 (l)
-
17N.2.hl.TZ0.7e:
State and explain the products of electrolysis of a concentrated aqueous solution of sodium chloride using inert electrodes. Your answer should include half-equations for the reaction at each electrode.
- 19N.2.hl.TZ0.6e(i): Calculate the cell potential at 298 K for the disproportionation reaction, in V, using...
- 19N.2.hl.TZ0.6e(ii): Comment on the spontaneity of the disproportionation reaction at 298 K.
-
19N.2.hl.TZ0.6c(iii):
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
-
19N.2.hl.TZ0.6c(iv):
Deduce the half-equation for the formation of the gas identified in (c)(iii).
-
17M.2.hl.TZ2.2b.ii:
Calculate the Gibbs free energy, ΔG θ, in kJ, which is released by the corrosion of 1 mole of iron. Use section 1 of the data booklet.
-
19N.2.hl.TZ0.6e(iv):
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
-
17M.2.hl.TZ1.3b.ii:
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
-
17M.1.hl.TZ1.30:
Which statement is correct for the overall reaction in a voltaic cell?
2AgNO3(aq) + Ni(s) → 2Ag(s) + Ni(NO3)2(aq) E θ= +1.06 V
A. Electrons flow from Ag electrode to Ni electrode.
B. Ni is oxidized to Ni2+ at the cathode (negative electrode).
C. Ag+ is reduced to Ag at the anode (positive electrode).
D. Ag has a more positive standard electrode potential value than Ni.
- 20N.1.hl.TZ0.30: Which conditions deposit the greatest mass of copper when solutions containing copper ions...
-
19N.1.hl.TZ0.32:
Three cells with platinum electrodes are connected in series to a DC power supply.
What is the ratio of moles formed at each cathode (negative electrode)?
-
16N.2.hl.TZ0.4i:
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
-
19M.2.hl.TZ1.6f(i):
Calculate the standard electrode potential, in V, when the Fe2+ (aq) | Fe (s) and Cu2+ (aq) | Cu (s) standard half-cells are connected at 298 K. Use section 24 of the data booklet.
-
17M.2.hl.TZ2.2b.i:
Corrosion of iron is similar to the processes that occur in a voltaic cell. The initial steps involve the following half-equations:
Fe2+(aq) + 2e– Fe(s)
O2(g) + H2O(l) + 2e– 2OH–(aq)
Calculate E θ, in V, for the spontaneous reaction using section 24 of the data booklet.
-
19M.2.hl.TZ2.4b(ii):
A scientist wants to investigate the catalytic properties of a thin layer of rhenium metal on a graphite surface.
Describe an electrochemical process to produce a layer of rhenium on graphite.
-
18M.2.hl.TZ1.6e:
Determine the loss in mass of one electrode if the mass of the other electrode increases by 0.10 g.
-
20N.2.hl.TZ0.4d(iii):
Calculate the standard free energy change, , in , for the cell using sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ1.7:
An aqueous solution of silver nitrate, AgNO3 (aq), can be electrolysed using platinum electrodes.
Formulate the half-equations for the reaction at each electrode during electrolysis.
Cathode (negative electrode):
Anode (positive electrode):
-
18M.2.hl.TZ2.4c:
Calculate the standard electrode potential, in V, for the BrO3−/Br− reduction half‑equation using section 24 of the data booklet.
-
19M.2.hl.TZ1.6f(ii):
Calculate ΔGθ, in kJ, for the spontaneous reaction in (f)(i), using sections 1 and 2 of the data booklet.
- 21M.1.hl.TZ1.30: Which gives the equation and cell potential of the spontaneous reaction?
-
21M.1.hl.TZ1.31:
What are the products when concentrated aqueous copper (II) chloride is electrolysed using platinum electrodes?
-
21M.2.hl.TZ1.3d:
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
- 21M.2.hl.TZ1.3e: The figure shows an apparatus that could be used to electroplate iron with zinc. Label the...
-
21M.1.hl.TZ2.31:
What happens to the mass of each copper electrode when aqueous copper(II) sulfate solution is electrolysed?
-
21M.1.hl.TZ2.30:
What would be the electrode potential, E⦵, of the Mn2+ (aq)|Mn (s) half-cell if Fe3+ (aq)|Fe2+ (aq) is used as the reference standard?
Mn2+ (aq) + 2e− Mn (s) E⦵ = −1.18 V
Fe3+ (aq) + e− Fe2+ (aq) E⦵ = +0.77 VA. −1.95 V
B. −0.41 V
C. +0.41 V
D. +1.95 V
-
18M.1.hl.TZ2.30:
Two cells undergoing electrolysis are connected in series.
If g of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
Ar(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
A.
B.
C.
D.
-
16N.2.hl.TZ0.4k:
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
-
20N.3.hl.TZ0.13a:
Write the balanced equation for the reaction in this voltaic cell.
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
-
21M.2.hl.TZ2.3c:
Calculate the cell potential using section 24 of the data booklet.
-
21M.2.hl.TZ2.3d:
Calculate the Gibbs free energy change, ΔG⦵, in kJ, for the cell, using section 1 of the data booklet.
-
19N.2.hl.TZ0.6e(iii):
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
-
18N.1.hl.TZ0.31:
Consider the standard electrode potentials:
Cr3+ (aq) + 3e− Cr (s) EΘ = −0.74 V
Hg2+ (aq) + 2e− Hg (l) EΘ = +0.85 V
What is the cell potential, in V, for the voltaic cell?
2Cr (s) + 3Hg2+ (aq) → 3Hg (l) + 2Cr3+ (aq)
A. −1.59
B. +0.11
C. +1.07
D. +1.59
-
20N.1.hl.TZ0.31:
Which statement is correct when a zinc spoon is electroplated with silver?
A. The cathode (negative electrode) is made of silver.
B. The anode (positive electrode) is the zinc spoon.
C. The anode (positive electrode) is made of silver.
D. The electrolyte is zinc sulfate solution.
-
20N.2.hl.TZ0.4d(ii):
Calculate the standard cell potential, in , for the cell at . Use section 24 of the data booklet
-
20N.2.hl.TZ0.6c:
The electron configuration of copper makes it a useful metal.
Copper plating can be used to improve the conductivity of an object.
State, giving your reason, at which electrode the object being electroplated should be placed.
-
20N.3.hl.TZ0.13c:
Predict, giving a reason, how an increase in temperature affects the potential of this cell.
-
17M.2.hl.TZ1.3c.ii:
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
-
18M.2.hl.TZ2.4b:
The change in the free energy for the reaction under standard conditions, ΔGΘ, is −514 kJ at 298 K.
Determine the value of EΘ, in V, for the reaction using sections 1 and 2 of the data booklet.
-
22M.1.hl.TZ1.31:
In the electrolysis apparatus shown, 0.59 g of Ni is deposited on the cathode of the first cell.
What is the mass of Ag deposited on the cathode of the second cell?
A. 0.54 gB. 0.59 g
C. 1.08 g
D. 2.16 g
-
22M.2.hl.TZ1.2b(ii):
Calculate the free energy change, ΔG⦵, in kJ, of the cell reaction. Use sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ2.4e(iii):
Predict, giving a reason, whether the reduction of ReO4− to [Re(OH)2]2+ would oxidize Fe2+ to Fe3+ in aqueous solution. Use section 24 of the data booklet.
-
22M.1.hl.TZ2.30:
Which E⦵ value, in V, for the reaction Mn (s) + Zn2+ (aq) → Mn2+ (aq) + Zn (s) can be deduced from the following equations?
Mn (s) + 2Ag+ (aq) → Mn2+ (aq) + 2Ag (s) E⦵ = 1.98 V
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) E⦵ = 1.10 V
Cu (s) + 2Ag+ (aq) → Cu2+ (aq) + 2Ag (s) E⦵ = 0.46 V
A. 0.42
B. 1.34
C. 2.62
D. 3.54
-
22M.2.hl.TZ2.3a(ii):
Determine, giving a reason, if iodine will also oxidize iron(II).
-
21N.1.hl.TZ0.31:
Which aqueous solutions produce oxygen gas during electrolysis?
I. Dilute CuCl2 (aq) with inert electrodes
II. Dilute FeSO4 (aq) with inert electrodes
III. Dilute CuCl2 (aq) with copper electrodesThe standard electrode potentials are provided in the table:
A. I and II onlyB. I and III only
C. II and III only
D. I, II and III
-
21N.2.hl.TZ0.8:
The standard electrode potential of zinc can be measured using a standard hydrogen electrode (SHE).
Draw and annotate the diagram to show the complete apparatus required to measure the standard electrode potential of zinc.

