| Date | November 2021 | Marks available | 1 | Reference code | 21N.1.hl.TZ0.23 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Determine | Question number | 23 | Adapted from | N/A |
Question
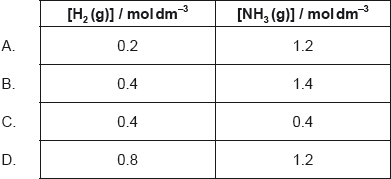
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) 2NO2 (g)
Which point shows the system at equilibrium?
Markscheme
B
Examiners report
[N/A]