| Date | November 2018 | Marks available | 1 | Reference code | 18N.1.hl.TZ0.16 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Question number | 16 | Adapted from | N/A |
Question
What are the signs of ΔHΘ and ΔSΘ for the reaction, which is spontaneous at low temperature and non-spontaneous at very high temperature?
ΔGΘ = ΔHΘ − TΔSΘ
SO3 (g) + CaO (s) → CaSO4 (s)
Markscheme
C
Examiners report
Syllabus sections
-
18M.1.hl.TZ2.17:
Which system has the most negative entropy change, ΔS, for the forward reaction?
A. N2(g) + 3H2(g) 2NH3(g)
B. CaCO3(s) → CaO(s) + CO2(g)
C. 2S2O32−(aq) + I2(aq) → S4O62−(aq) + 2I–(aq)
D. H2O(l) → H2O(g)
-
17N.2.hl.TZ0.5c:
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
-
19M.1.hl.TZ2.17:
Which change has the greatest increase in entropy?
A. CO2 (s) → CO2 (g)
B. CO2 (g) → CO2 (l)
C. CO2 (g) → CO2 (s)
D. CO2 (l) → CO2 (s)
-
17N.1.hl.TZ0.17:
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
- 22M.2.hl.TZ1.3c(iv): Outline, with reference to the reaction equation, why this sign for the entropy change is...
-
22M.1.hl.TZ1.17:
In which reaction does entropy decrease?
A. NaCl (s) → NaCl (aq)
B. Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
C. NH3 (g) + HCl (g) → NH4Cl (s)
D. CuCO3 (s) → CuO (s) + CO2 (g)
- 22M.1.hl.TZ2.15: What are the signs of ΔH and ΔS for a reaction that is non-spontaneous at low temperatures...
-
18M.2.hl.TZ1.3c.v:
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
- 18M.1.hl.TZ1.17: Which statement is correct? A. If ΔH < 0, reaction is always spontaneous. B. If...
-
22M.2.hl.TZ2.4d(iii):
Calculate the Gibbs free energy change, ΔG⦵, in kJ mol−1, for the reaction at 298 K. Use section 1 of the data booklet.
-
22M.2.hl.TZ2.4d(i):
Calculate the entropy change of reaction, ΔS⦵, in J K−1 mol−1.
-
18N.2.hl.TZ0.5d:
Predict, giving your reasons, whether the forward reaction is endothermic or exothermic. Use your answers to (b) and (c).
- 18N.2.hl.TZ0.5b: Predict, giving your reason, the sign of the standard entropy change of the forward reaction.
-
17M.2.hl.TZ2.9b.ii:
The standard enthalpy change, ΔH θ, for the hydrogenation of propene is –124.4 kJ mol–1. Predict the temperature above which the hydrogenation reaction is not spontaneous.
-
17M.2.hl.TZ1.4c.i:
Calculate the standard entropy change, , of the reaction, in , using the values given.
-
20N.2.hl.TZ0.3c:
Predict, giving a reason, whether the entropy change, , for this reaction is negative or positive.
-
17N.2.hl.TZ0.5b:
Calculate the standard entropy change for this reaction using the following data.
- 17M.1.hl.TZ1.17: Which combination of ΔH θ and ΔS θ will result in a non-spontaneous reaction at all...
-
17M.2.hl.TZ2.9b.i:
Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for the hydrogenation of propene.
-
19N.2.hl.TZ0.4a(v):
Comment on the spontaneity of the reaction at 298 K.
- 19N.2.hl.TZ0.6e(v): Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the...
-
19M.2.hl.TZ2.2g(i):
Determine the standard entropy change, in J K−1, for the decomposition of dinitrogen monoxide.
2N2O (g) → 2N2 (g) + O2 (g)
-
18M.2.hl.TZ2.5e:
Determine the temperature, in K, above which the reaction becomes spontaneous.
-
19N.2.hl.TZ0.4a(iv):
Calculate the standard Gibbs free energy change, , in kJ mol−1, for the first dissociation of citric acid at 298 K, using section 1 of the data booklet.
-
18M.2.hl.TZ2.5d:
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
-
19N.1.hl.TZ0.17:
Which reaction has the greatest increase in entropy of the system?
A. HCl (g) + NH3 (g) → NH4Cl (s)
B. (NH4)2Cr2O7 (s) → Cr2O3 (s) + N2 (g) + 4H2O (g)
C. CaCO3 (s) → CaO (s) + CO2 (g)
D. I2 (g) → I2 (s)
-
18M.2.hl.TZ1.3c.iv:
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
-
19M.1.hl.TZ1.16:
Which is correct for the reaction H2O (g) → H2O (l) ?
A. Enthalpy increases and entropy increases.
B. Enthalpy decreases and entropy increases.
C. Enthalpy increases and entropy decreases.
D. Enthalpy decreases and entropy decreases.
-
19N.2.hl.TZ0.6e(vi):
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
-
20N.2.hl.TZ0.3e:
Calculate the standard Gibbs free energy change, , in , for the reaction at 5 °C, using your answers to (b) and (d). Use section 1 of the data booklet.
(If you did not obtain an answer to (b) or (d) use values of and respectively, although these are not the correct answers.)
-
16N.2.hl.TZ0.1b:
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
-
21M.1.hl.TZ1.16:
The table shows the variation of standard Gibbs energy with temperature for a reversible reaction.
What can be concluded about the reaction?
A. Equilibrium shifts left as temperature increases.
B. The forward reaction is more spontaneous below 300 K.
C. Entropy is higher in the products than in the reactants.
D. Kc decreases as temperature increases.
-
19M.2.hl.TZ2.2g(ii):
Dinitrogen monoxide has a positive standard enthalpy of formation, ΔHfθ.
Deduce, giving reasons, whether altering the temperature would change the spontaneity of the decomposition reaction.
-
21M.2.hl.TZ1.4e(ii):
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
-
17N.1.hl.TZ0.16:
What is the standard enthalpy of formation, in kJ mol–1, of IF (g)?
IF7 (g) + I2 (s) → IF5 (g) + 2IF (g) ΔH = –89 kJ
ΔH (IF7) = –941 kJ mol–1
ΔH (IF5) = –840 kJ mol–1
A. –190
B. –95
C. +6
D. +95
-
21M.1.hl.TZ2.17:
Which change results in the largest negative value of ΔS?
A. C2H5OH (l) + SOCl2 (l) → C2H5Cl (l) + SO2 (g) + HCl (g)
B. CaCO3 (s) → CaO (s) + CO2 (g)
C. H2O (l) → H2O (s)
D. NH3 (g) + HCl (g) → NH4Cl (s)
-
22M.2.hl.TZ2.4d(ii):
Predict, giving a reason, how the value of the ΔS⦵reaction would be affected if (g) were used as a reactant.
-
17M.2.hl.TZ1.4c.iii:
Use your answers to (c)(i) and (c)(ii), to determine the temperature, in °C, at which the decomposition of liquid tetracarbonylnickel to nickel and carbon monoxide becomes favourable.
(If you did not get answers to (c)(i) and (c)(ii), use and respectively but these are not the correct answers.) -
21M.2.hl.TZ2.1b(ii):
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
-
21M.2.hl.TZ2.1b(iii):
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
-
20N.1.hl.TZ0.17:
Which reaction becomes more spontaneous as temperature increases?
A.
B.
C.
D.
-
18M.2.hl.TZ2.5c:
The table lists standard entropy, SΘ, values.
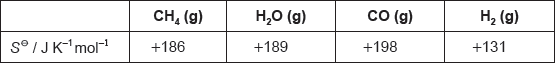
Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
-
20N.2.hl.TZ0.2f(ii):
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
-
17M.2.hl.TZ1.3c.ii:
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
-
17M.2.hl.TZ1.4c.ii:
Calculate a value for in kJ.
-
22M.2.hl.TZ1.3c(iii):
Calculate the entropy change for the Haber–Bosch process, in J mol–1 K–1 at 298 K. Use your answer to (b)(i) and section 1 of the data booklet.
-
20N.2.hl.TZ0.3d:
Calculate for the reaction in , using section 12 of the data booklet.
The standard molar entropy for oxygen gas is .
- 22M.1.hl.TZ2.17: Which term in the expression ΔG⦵ = ΔH⦵ − TΔS⦵ is an indirect measure of the entropy change of...
- 21M.2.hl.TZ1.4e(iii): Justify the sign of ΔS with reference to the equation.
- 21M.2.hl.TZ1.4e(iv): Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the...
-
21N.2.hl.TZ0.3c(ii):
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
- 21N.1.hl.TZ0.17: In which of the following situations is the forward reaction spontaneous? A. The...
-
21N.2.hl.TZ0.3c(iii):
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.

