| Date | May 2017 | Marks available | 2 | Reference code | 17M.2.hl.TZ2.3 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 3 | Adapted from | N/A |
Question
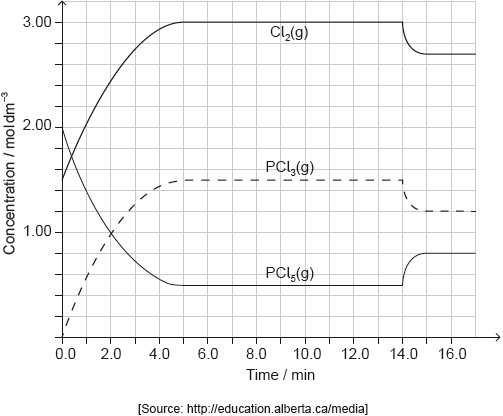
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
Markscheme
Lewis structure:
Molecular geometry:
trigonal/triangular pyramidal
Bond angles:
< 109.5°
Penalize missing lone pairs once only between this question and 4(b)(ii).
Accept any combination of lines, dots or crosses to represent electrons.
Do not apply ECF.
Do not accept answer equal to or less than 90°.
Literature value is 100.1°.
[3 marks]