DP Chemistry Questionbank

Topic 17: Equilibrium
Description
[N/A]Directly related questions
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and respectively.
X + 2Y 2W + Z
What is the value of the equilibrium constant, Kc?
A.
B.
C. 2
D. 8
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
-
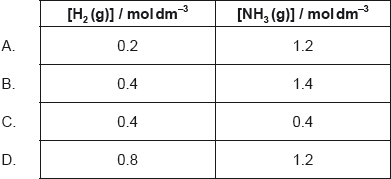
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
Sub sections and their related questions
17.1 The equilibrium law
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and respectively.
X + 2Y 2W + Z
What is the value of the equilibrium constant, Kc?
A.
B.
C. 2
D. 8
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
-
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?

-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
