| Date | May 2021 | Marks available | 1 | Reference code | 21M.1.hl.TZ1.23 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Solve | Question number | 23 | Adapted from | N/A |
Question
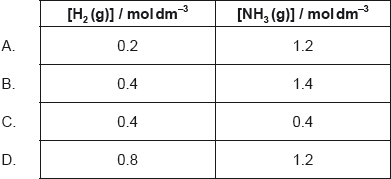
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
Markscheme
D
Examiners report
[N/A]