| Date | May 2017 | Marks available | 1 | Reference code | 17M.1.hl.TZ2.17 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | State | Question number | 17 | Adapted from | N/A |
Question
Which ion’s hydration energy is the most exothermic?
A. Li+
B. Na+
C. Br–
D. I–
Markscheme
A
Examiners report
Syllabus sections
-
19M.1.hl.TZ2.16:
Which equation represents lattice enthalpy?
A. NaCl (g) → Na+ (g) + Cl− (g)
B. NaCl (s) → Na+ (g) + Cl− (g)
C. NaCl (s) → Na+ (aq) + Cl− (aq)
D. NaCl (s) → Na+ (s) + Cl− (s)
- 17N.1.hl.TZ0.15: Which statements are correct for ionic compounds? I. Lattice energy increases as ionic...
-
19M.1.hl.TZ1.17:
Which equation represents the standard enthalpy of atomization of bromine, Br2?
A. Br2 (l) → Br (g)
B. Br2 (l) → 2Br (g)
C. Br2 (l) → 2Br (l)
D. Br2 (l) → Br (l)
-
22M.1.hl.TZ2.14:
Which equation represents hydration enthalpy?
A. Na+ (g) → Na+ (aq)
B. Na+ (aq) → Na+ (g)
C. NaCl (s) → NaCl (aq)
D. NaCl (aq) → NaCl (s)
-
18M.1.hl.TZ2.16:
Which value represents the lattice enthalpy, in kJ mol−1, of strontium chloride, SrCl2?
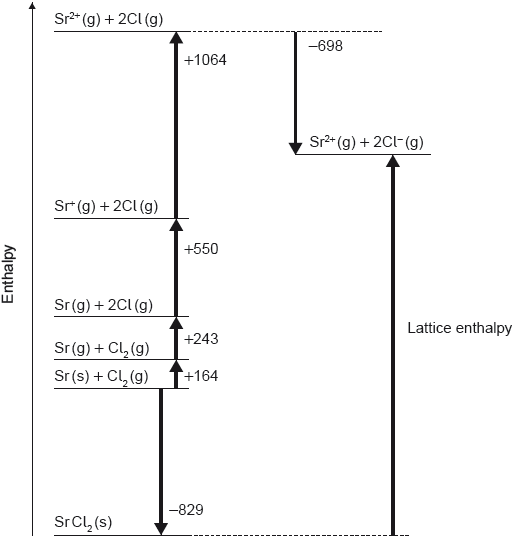
A. – (–829) + 164 + 243 + 550 + 1064 – (–698)
B. –829 + 164 + 243 + 550 + 1064 – 698
C. – (–829) + 164 + 243 + 550 + 1064 – 698
D. –829 + 164 + 243 + 550 + 1064 – (–698)
-
18M.1.hl.TZ1.16:
What is the enthalpy of solution of MgF2(s) in kJ mol−1?
Lattice enthalpy of MgF2(s) = 2926 kJ mol−1
Hydration enthalpy of Mg2+(g) = −1963 kJ mol−1
Hydration enthalpy of F−(g) = −504 kJ mol−1
A. 2926 − 1963 + 2(−504)
B. 2926 − 1963 − 504
C. −2926 − (−1963) − (−504)
D. −2926 − (−1963) − 2(−504)
- 16N.1.hl.TZ0.18: Which represents the enthalpy change of hydration of the chloride ion?
- 18N.2.hl.TZ0.4d: Predict, giving your reasons, whether Mn2+ or Fe2+ is likely to have a more exothermic...
-
17N.1.hl.TZ0.18:
Which equation represents the lattice enthalpy of magnesium sulfide?
A. MgS (s) → Mg (g) + S (g)
B. MgS (s) → Mg+ (g) + S– (g)
C. MgS (s) → Mg2+ (g) + S2– (g)
D. MgS (s) → Mg (s) + S (s)
- 22M.1.hl.TZ1.16: Which compound has the largest value of lattice enthalpy? A. Na2O B. K2O C. Na2S D. K2S
-
17M.1.hl.TZ1.16:
Which equation represents enthalpy of hydration?
A. Na(g) → Na+(aq) + e−
B. Na+(g) → Na+(aq)
C. NaCl(s) → Na+(g) + Cl−(g)
D. NaCl(s) → Na+(aq) + Cl−(aq)
- 16N.1.hl.TZ0.19: Which ionic compound has the largest value of lattice enthalpy? A. MgS B. MgO C. CaBr2...
-
17M.1.hl.TZ2.16:
The Born-Haber cycle for potassium oxide is shown below:
Which expression represents the lattice enthalpy in kJ mol–1?
A. –361 + 428 + 838 + 612
B. –(–361) + 428 + 838 + 612
C. –361 + 428 + 838 – 612
D. –(–361) + 428 + 838 – 612
-
20N.1.hl.TZ0.16:
Which combination gives the standard hydration enthalpy of ?
A.
B.
C.
D.
-
18N.1.hl.TZ0.17:
Which change is exothermic?
A. Cl2 (g) → Cl (g)
B. K (g) → K+ (g) + e−
C. KCl (s) → K+ (g) + Cl− (g)
D. Cl (g) + e− → Cl− (g)
-
19N.2.hl.TZ0.6d:
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
-
21M.2.hl.TZ2.5a(ii):
Deduce the change in enthalpy, ΔH, in kJ, when 56.00 g of ethanol is burned. Use section 13 in the data booklet.
-
19N.1.hl.TZ0.18:
What is the order of increasing (more exothermic) enthalpy of hydration?
Xn+ (g) → Xn+ (aq)
A. Ca2+, Mg2+, K+, Na+
B. Na+, K+, Mg2+, Ca2+
C. K+, Na+, Ca2+, Mg2+
D. Mg2+, Ca2+, Na+, K+
-
19M.2.hl.TZ1.3d(iii):
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
-
19M.2.hl.TZ1.3d(ii):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.) -
19M.2.hl.TZ1.3d(i):
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1O2(g) → O2- (g):
Na (s) → Na+ (g):
-
21M.1.hl.TZ2.16:
Which represents electron affinity?
A. Al2+ (g) → Al3+ (g) + e−
B. C (g) + e− → C− (g)
C. Cl2 (g) → 2Cl (g)
D. S (s) → S+ (g) + e−
-
21M.1.hl.TZ1.17:
Which substance has the highest lattice enthalpy?
A.
B.
C.
D.
-
21N.1.hl.TZ0.16:
Consider the Born–Haber cycle for the formation of sodium oxide:
What is the lattice enthalpy, in kJ mol−1, of sodium oxide?
A. 414 + 2(108) + 249 + 2(496) − 141 + 790B. 414 + 2(108) + 249 + 2(496) + 141 + 790
C. −414 + 2(108) + 249 + 2(496) − 141 + 790
D. −414 − 2(108) − 249 − 2(496) + 141 − 790

