| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.hl.TZ1.23 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Solve | Question number | 23 | Adapted from | N/A |
Question
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?
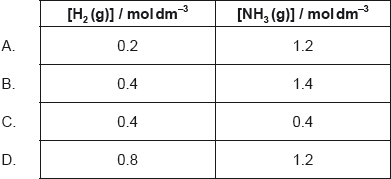
Markscheme
B
Examiners report
[N/A]

