| Date | November 2017 | Marks available | 1 | Reference code | 17N.2.hl.TZ0.6 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Determine | Question number | 6 | Adapted from | N/A |
Question
Many reactions are in a state of equilibrium.
The following reaction was allowed to reach equilibrium at 761 K.
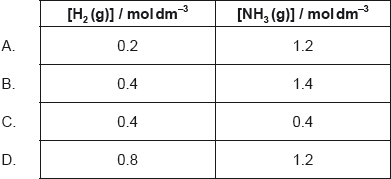
H2 (g) + I2 (g) 2HI (g) ΔHθ < 0
The pH of 0.010 mol dm–3 carbonic acid, H2CO3 (aq), is 4.17 at 25 °C.
H2CO3 (aq) + H2O (l) HCO3– (aq) + H3O+ (aq).
State the equilibrium constant expression, Kc , for this reaction.
The following equilibrium concentrations in mol dm–3 were obtained at 761 K.
Calculate the value of the equilibrium constant at 761 K.
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
Calculate Kb for HCO3– acting as a base.
Markscheme
Kc =
45.6
ΔGθ = «– RT ln K = – (0.00831 kJ K−1 mol−1 x 761 K x ln 45.6) =» – 24.2 «kJ»
[H3O+] = 6.76 x 10–5 «mol dm–3»
Ka =
4.6 x 10–7
Accept 4.57 x 10–7
Award [3] for correct final answer.
« =» 2.17 x 10–8
OR
« =» 2.19 x 10–8