DP Chemistry Questionbank

Topic 18: Acids and bases
Description
[N/A]Directly related questions
-
16N.1.hl.TZ0.28:
Which mixture is a buffer solution?
A. 25 cm3 of 0.10 mol dm-3 NH3 (aq) and 50 cm3 of 0.10 mol dm-3 HCl (aq)
B. 50 cm3 of 0.10 mol dm-3 NH3 (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
C. 25 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
D. 50 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
-
16N.2.hl.TZ0.7a:
Calculate the pH of 0.0100 mol dm–3 methanoic acid stating any assumption you make. Ka = 1.6 × 10–4.
-
16N.1.hl.TZ0.29:
Which salt solution has the highest pH?
A. NH4Cl
B. Ca(NO3)2
C. Na2CO3
D. K2SO4
-
16N.2.hl.TZ0.7b:
(i) Sketch a graph of pH against volume of a strong base added to a weak acid showing how you would determine pKa for the weak acid.
(ii) Explain, using an equation, why the pH increases very little in the buffer region when a small amount of alkali is added.
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
- 20N.2.hl.TZ0.5b(ii): State a suitable indicator for this titration. Use section 22 of the data booklet
-
20N.2.hl.TZ0.5e:
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
-
20N.2.hl.TZ0.5b(iii):
Suggest, giving a reason, which point on the curve is considered a buffer region.
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
20N.2.hl.TZ0.5b(i):
Identify the major species, other than water and potassium ions, at these points.
-
17M.1.hl.TZ1.27:
A buffer is produced by mixing 20.0 cm3 of 0.10 mol dm−3 ethanoic acid, CH3COOH(aq), with 0.10 mol dm−3 sodium hydroxide, NaOH(aq).
What is the volume of NaOH required and the pH of the buffer?
-
17M.2.hl.TZ1.2e:
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
-
17M.2.hl.TZ1.5d.i:
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
-
17M.2.hl.TZ1.5d.ii:
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
- 17M.1.hl.TZ2.26: Which type of bond is formed when a Lewis acid reacts with a Lewis base? A. Covalent B. ...
- 17M.1.hl.TZ2.27: What is the order of increasing acidity of the following acids? A. chloroethanoic <...
-
17M.2.hl.TZ2.8b.iv:
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
-
17M.2.hl.TZ2.8b.vi:
Deduce the pKa for this acid.
-
17M.2.hl.TZ2.8c:
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
-
17N.1.hl.TZ0.27:
Which indicator is appropriate for the acid-base titration shown below?
A. Thymol blue (pKa = 1.5)
B. Methyl orange (pKa = 3.7)
C. Bromophenol blue (pKa = 4.2)
D. Phenolphthalein (pKa = 9.6) - 17N.1.hl.TZ0.26: Which of the following will form a buffer solution if combined in appropriate molar ratios? A....
-
17N.2.hl.TZ0.6c.ii:
Calculate Kb for HCO3– acting as a base.
- 17N.2.hl.TZ0.3e: Describe, in terms of acid-base theories, the type of reaction that takes place between the...
-
17N.2.hl.TZ0.6c.i:
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
- 21M.1.sl.TZ1.19: Which is amphiprotic? A. NH4+ B. PO43− C. H2O D. H3O+
- 21M.1.hl.TZ1.27: Which combination will produce an alkaline buffer in water? A. 0.10 mol NH3 and 0.05 mol...
- 21M.1.hl.TZ2.26: Which is correct? A. Electrophiles are Brønsted–Lowry acids. B. Nucleophiles are...
- 21M.1.hl.TZ2.27: Which compound is acidic in aqueous solution? A. KBr B. CH3COONa C. NH4Cl D. Na2CO3
- 21M.2.hl.TZ1.3g: Transition metals like iron can form complex ions. Discuss the bonding between transition metals...
-
21M.2.hl.TZ1.8a:
Calculate the pH of 0.00100 mol dm–3 propanoic acid solution. Use section 21 of the data booklet.
-
21M.2.hl.TZ1.8b:
Sketch the general shape of the variation of pH when 50 cm3 of 0.001 mol dm–3 NaOH (aq) is gradually added to 25 cm3 of 0.001 mol dm–3 CH3CH2COOH (aq).
- 21M.2.hl.TZ2.5d(i): Sketch the titration curve of methanoic acid with sodium hydroxide, showing how you would...
-
21M.2.hl.TZ2.5e:
Determine the concentration of methanoic acid in a solution of pH = 4.12. Use section 21 of the data booklet.
-
21M.2.hl.TZ2.5f:
Identify if aqueous solutions of the following salts are acidic, basic, or neutral.
-
21M.2.hl.TZ2.5d(ii):
Identify an indicator that could be used for the titration in 5(d)(i), using section 22 of the data booklet.
-
18M.2.hl.TZ2.2d.ii:
Calculate the pH of 0.100 mol dm−3 aqueous ethanoic acid.
Ka = 1.74 × 10−5
- 18M.1.hl.TZ2.26: Which is an example of a Lewis base? A. an electrophile B. BF3 C. CH4 D. a...
- 18M.1.hl.TZ1.27: Which combination of acid and base is most likely to have a pH of 8.5 at the equivalence point in...
- 18M.1.hl.TZ1.26: Which statements are correct? I. Lewis bases can act as nucleophiles. II....
- 18M.1.hl.TZ2.27: What is the order of increasing acidity? A. HClO < CH3CH2COOH < HF < HIO3 B. ...
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
-
18M.2.hl.TZ1.5d:
Determine the pH of 0.010 mol dm−3 2,2-dimethylpropanoic acid solution.
Ka (2,2-dimethylpropanoic acid) = 9.333 × 10−6
-
18M.2.hl.TZ1.5e:
Explain, using appropriate equations, how a suitably concentrated solution formed by the partial neutralization of 2,2-dimethylpropanoic acid with sodium hydroxide acts as a buffer solution.
-
18M.2.hl.TZ2.2d.i:
The graph represents the titration of 25.00 cm3 of 0.100 mol dm−3 aqueous ethanoic acid with 0.100 mol dm−3 aqueous sodium hydroxide.
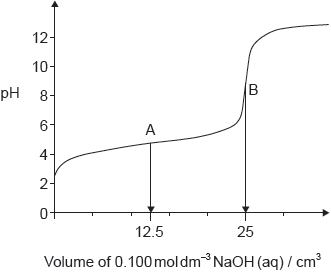
Deduce the major species, other than water and sodium ions, present at points A and B during the titration.
-
18M.2.hl.TZ2.2d.iv:
Predict whether the pH of an aqueous solution of ammonium chloride will be greater than, equal to or less than 7 at 298 K.
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
-
21N.2.hl.TZ0.11b:
The concentration of excess sodium hydroxide was 0.362 mol dm−3. Calculate the pH of the solution at the end of the experiment.
-
21N.2.hl.TZ0.11c:
Sketch the neutralisation curve obtained and label the equivalence point.
- 21N.2.hl.TZ0.10e: Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
-
21N.2.hl.TZ0.11a:
Calculate the initial pH before any sodium hydroxide was added, using section 21 of the data booklet.
- 18N.1.hl.TZ0.26: Which species is not a Lewis base? A. OH− B. NH4+ C. H2O D. PH3
- 18N.2.hl.TZ0.9d: State, giving your reason, whether the hydroxide ion acts as a Lewis acid, a Lewis base, or...
-
18N.2.hl.TZ0.6b.ii:
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
-
18N.1.hl.TZ0.27:
An indicator, HIn, has a pKa of 5.1.
HIn (aq) H+ (aq) + In− (aq)
colour A colour B
Which statement is correct?
A. At pH = 7, colour B would be observed
B. At pH = 3, colour B would be observed
C. At pH = 7, [HIn] = [In−]
D. At pH = 3, [HIn] < [In−] - 18N.1.hl.TZ0.25: What is the order of increasing pH for the following solutions of the same concentration? A. ...
-
18N.2.hl.TZ0.6c:
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
- 22M.1.hl.TZ1.26: Which statement explains the Lewis acid–base nature of the chloride ion in this reaction? C2H5+...
- 22M.1.hl.TZ1.27: In which set are the salts arranged in order of increasing pH? A. HCOONH4 < KBr < NH4Br...
- 22M.1.hl.TZ2.26: A weak base is titrated with a strong acid. Which value of pKb can be estimated from this...
- 22M.2.hl.TZ1.1d(ii): Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that...
-
22M.2.hl.TZ1.4d:
Magnesium salts form slightly acidic solutions owing to equilibria such as:
Mg2+ (aq) + H2O (l) Mg(OH)+ (aq) + H+ (aq)
Comment on the role of Mg2+ in forming the Mg(OH)+ ion, in acid-base terms.
-
22M.2.hl.TZ1.4c(ii):
Calculate the concentration, in mol dm–3, of ammonia molecules in the solution with pH = 9.3. Use section 21 of the data booklet.
- 22M.2.hl.TZ2.7a(i): State why NH3 is a Lewis base.
-
22M.2.hl.TZ2.7a(ii):
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
- 22M.2.hl.TZ2.7a(iii): Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer...
- 22M.1.hl.TZ2.27: Which species are both Lewis and Brønsted–Lowry bases? I. CN−II. OH−III. NH3 A. I and II...
-
22M.2.hl.TZ1.4c(iii):
An aqueous solution containing high concentrations of both NH3 and NH4+ acts as an acid-base buffer solution as a result of the equilibrium:
NH3 (aq) + H+ (aq) NH4+ (aq)
Referring to this equilibrium, outline why adding a small volume of strong acid would leave the pH of the buffer solution almost unchanged.
-
19M.2.hl.TZ1.5d(ii):
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
-
19M.2.hl.TZ1.5d(i):
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
-
19M.2.hl.TZ1.5d(iii):
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
-
19M.2.hl.TZ2.5c:
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
-
19M.2.hl.TZ2.5f:
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
-
19M.1.hl.TZ1.27:
Which has the strongest conjugate base?
A. HCOOH (Ka = 1.8 × 10−4)
B. HNO2 (Ka = 7.2 × 10−4)
C. HCN (Ka = 6.2 × 10−10)
D. HIO3 (Ka = 1.7 × 10−1)
- 19M.1.hl.TZ1.26: Which is a Lewis acid but not a Brønsted−Lowry acid? A. AlCl3 B. CH3CO2H C. HF D. CCl4
-
19M.1.hl.TZ2.26:
Where is the buffer region for the titration of a weak acid with a strong base?
-
19M.1.hl.TZ2.27:
The following equation represents the dissociation of water at 25 °C.
2H2O (l) H3O+ (aq) + OH− (aq) ΔH = +56 kJ
Which changes occur as the temperature increases?
A. [H3O+] increases and pH will decrease.
B. [H3O+] decreases and pH will increase.
C. [H3O+] increases and pH will increase.
D. [H3O+] decreases and pH will decrease.
-
19N.2.hl.TZ0.6f(iv):
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
- 19N.2.hl.TZ0.5b(i): Identify the most suitable indicator for the titration using section 22 of the data booklet.
- 19N.1.hl.TZ0.28: What is the order, in increasing pH, of the following solutions of equal concentration? A....
- 19N.2.hl.TZ0.5a: A sample of ethanoic acid was titrated with sodium hydroxide solution, and the following pH curve...
- 19N.1.hl.TZ0.27: Which can act as a Lewis acid but not a Brønsted–Lowry acid? A. BF3 B. H2O C. NF3 D. NH3
-
19N.2.hl.TZ0.5b(ii):
Describe, using a suitable equation, how the buffer solution formed during the titration resists pH changes when a small amount of acid is added.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
Sub sections and their related questions
18.1 Lewis acids and bases
-
17M.2.hl.TZ1.2e:
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
- 17M.1.hl.TZ2.26: Which type of bond is formed when a Lewis acid reacts with a Lewis base? A. Covalent B. ...
- 17N.2.hl.TZ0.3e: Describe, in terms of acid-base theories, the type of reaction that takes place between the...
- 18M.1.hl.TZ1.26: Which statements are correct? I. Lewis bases can act as nucleophiles. II....
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
- 18M.1.hl.TZ2.26: Which is an example of a Lewis base? A. an electrophile B. BF3 C. CH4 D. a...
- 18N.1.hl.TZ0.26: Which species is not a Lewis base? A. OH− B. NH4+ C. H2O D. PH3
- 18N.2.hl.TZ0.9d: State, giving your reason, whether the hydroxide ion acts as a Lewis acid, a Lewis base, or...
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
- 19M.1.hl.TZ1.26: Which is a Lewis acid but not a Brønsted−Lowry acid? A. AlCl3 B. CH3CO2H C. HF D. CCl4
-
19N.2.hl.TZ0.6f(iv):
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
- 19N.1.hl.TZ0.27: Which can act as a Lewis acid but not a Brønsted–Lowry acid? A. BF3 B. H2O C. NF3 D. NH3
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
- 21M.1.sl.TZ1.19: Which is amphiprotic? A. NH4+ B. PO43− C. H2O D. H3O+
- 21M.1.hl.TZ2.26: Which is correct? A. Electrophiles are Brønsted–Lowry acids. B. Nucleophiles are...
- 21M.2.hl.TZ1.3g: Transition metals like iron can form complex ions. Discuss the bonding between transition metals...
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
- 21N.2.hl.TZ0.10e: Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
- 22M.1.hl.TZ1.26: Which statement explains the Lewis acid–base nature of the chloride ion in this reaction? C2H5+...
- 22M.1.hl.TZ2.27: Which species are both Lewis and Brønsted–Lowry bases? I. CN−II. OH−III. NH3 A. I and II...
-
22M.2.hl.TZ1.4d:
Magnesium salts form slightly acidic solutions owing to equilibria such as:
Mg2+ (aq) + H2O (l) Mg(OH)+ (aq) + H+ (aq)
Comment on the role of Mg2+ in forming the Mg(OH)+ ion, in acid-base terms.
- 22M.2.hl.TZ2.7a(i): State why NH3 is a Lewis base.
18.2 Calculations involving acids and bases
-
16N.2.hl.TZ0.7a:
Calculate the pH of 0.0100 mol dm–3 methanoic acid stating any assumption you make. Ka = 1.6 × 10–4.
-
17M.2.hl.TZ1.5d.i:
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
- 17M.1.hl.TZ2.27: What is the order of increasing acidity of the following acids? A. chloroethanoic <...
-
17M.2.hl.TZ2.8c:
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
-
17N.2.hl.TZ0.6c.i:
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
-
17N.2.hl.TZ0.6c.ii:
Calculate Kb for HCO3– acting as a base.
-
18M.2.hl.TZ1.5d:
Determine the pH of 0.010 mol dm−3 2,2-dimethylpropanoic acid solution.
Ka (2,2-dimethylpropanoic acid) = 9.333 × 10−6
- 18M.1.hl.TZ2.27: What is the order of increasing acidity? A. HClO < CH3CH2COOH < HF < HIO3 B. ...
-
18M.2.hl.TZ2.2d.ii:
Calculate the pH of 0.100 mol dm−3 aqueous ethanoic acid.
Ka = 1.74 × 10−5
-
18N.2.hl.TZ0.6b.ii:
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
-
19M.2.hl.TZ2.5c:
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
-
19M.1.hl.TZ1.27:
Which has the strongest conjugate base?
A. HCOOH (Ka = 1.8 × 10−4)
B. HNO2 (Ka = 7.2 × 10−4)
C. HCN (Ka = 6.2 × 10−10)
D. HIO3 (Ka = 1.7 × 10−1)
-
19M.1.hl.TZ2.27:
The following equation represents the dissociation of water at 25 °C.
2H2O (l) H3O+ (aq) + OH− (aq) ΔH = +56 kJ
Which changes occur as the temperature increases?
A. [H3O+] increases and pH will decrease.
B. [H3O+] decreases and pH will increase.
C. [H3O+] increases and pH will increase.
D. [H3O+] decreases and pH will decrease.
-
19N.3.hl.TZ0.10b(iii):
Calculate the ratio of [A−] : [HA] in a buffer of pH 6.0 given that pKa for the acid is 4.83, using section 1 of the data booklet.
- 19N.1.hl.TZ0.28: What is the order, in increasing pH, of the following solutions of equal concentration? A....
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
20N.2.hl.TZ0.5e:
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
- 21M.1.hl.TZ1.27: Which combination will produce an alkaline buffer in water? A. 0.10 mol NH3 and 0.05 mol...
-
21M.2.hl.TZ1.8a:
Calculate the pH of 0.00100 mol dm–3 propanoic acid solution. Use section 21 of the data booklet.
-
21M.2.hl.TZ2.5e:
Determine the concentration of methanoic acid in a solution of pH = 4.12. Use section 21 of the data booklet.
-
21N.2.hl.TZ0.11a:
Calculate the initial pH before any sodium hydroxide was added, using section 21 of the data booklet.
-
21N.2.hl.TZ0.11b:
The concentration of excess sodium hydroxide was 0.362 mol dm−3. Calculate the pH of the solution at the end of the experiment.
-
22M.2.hl.TZ1.4c(ii):
Calculate the concentration, in mol dm–3, of ammonia molecules in the solution with pH = 9.3. Use section 21 of the data booklet.
-
22M.2.hl.TZ2.7a(ii):
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
18.3 pH curves
-
16N.1.hl.TZ0.28:
Which mixture is a buffer solution?
A. 25 cm3 of 0.10 mol dm-3 NH3 (aq) and 50 cm3 of 0.10 mol dm-3 HCl (aq)
B. 50 cm3 of 0.10 mol dm-3 NH3 (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
C. 25 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
D. 50 cm3 of 0.10 mol dm-3 NaOH (aq) and 25 cm3 of 0.10 mol dm-3 HCl (aq)
-
16N.1.hl.TZ0.29:
Which salt solution has the highest pH?
A. NH4Cl
B. Ca(NO3)2
C. Na2CO3
D. K2SO4
-
16N.2.hl.TZ0.7b:
(i) Sketch a graph of pH against volume of a strong base added to a weak acid showing how you would determine pKa for the weak acid.
(ii) Explain, using an equation, why the pH increases very little in the buffer region when a small amount of alkali is added.
-
17M.1.hl.TZ1.27:
A buffer is produced by mixing 20.0 cm3 of 0.10 mol dm−3 ethanoic acid, CH3COOH(aq), with 0.10 mol dm−3 sodium hydroxide, NaOH(aq).
What is the volume of NaOH required and the pH of the buffer?
-
17M.2.hl.TZ1.5d.ii:
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
-
17M.2.hl.TZ2.8b.iv:
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
-
17M.2.hl.TZ2.8b.vi:
Deduce the pKa for this acid.
- 17N.1.hl.TZ0.26: Which of the following will form a buffer solution if combined in appropriate molar ratios? A....
-
17N.1.hl.TZ0.27:
Which indicator is appropriate for the acid-base titration shown below?
A. Thymol blue (pKa = 1.5)
B. Methyl orange (pKa = 3.7)
C. Bromophenol blue (pKa = 4.2)
D. Phenolphthalein (pKa = 9.6) - 18M.1.hl.TZ1.27: Which combination of acid and base is most likely to have a pH of 8.5 at the equivalence point in...
-
18M.2.hl.TZ1.5e:
Explain, using appropriate equations, how a suitably concentrated solution formed by the partial neutralization of 2,2-dimethylpropanoic acid with sodium hydroxide acts as a buffer solution.
-
18M.2.hl.TZ2.2d.i:
The graph represents the titration of 25.00 cm3 of 0.100 mol dm−3 aqueous ethanoic acid with 0.100 mol dm−3 aqueous sodium hydroxide.

Deduce the major species, other than water and sodium ions, present at points A and B during the titration.
-
18M.2.hl.TZ2.2d.iv:
Predict whether the pH of an aqueous solution of ammonium chloride will be greater than, equal to or less than 7 at 298 K.
- 18N.1.hl.TZ0.25: What is the order of increasing pH for the following solutions of the same concentration? A. ...
-
18N.1.hl.TZ0.27:
An indicator, HIn, has a pKa of 5.1.
HIn (aq) H+ (aq) + In− (aq)
colour A colour B
Which statement is correct?
A. At pH = 7, colour B would be observed
B. At pH = 3, colour B would be observed
C. At pH = 7, [HIn] = [In−]
D. At pH = 3, [HIn] < [In−] -
18N.2.hl.TZ0.6c:
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
-
19M.2.hl.TZ1.5d(i):
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
-
19M.2.hl.TZ1.5d(ii):
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
-
19M.2.hl.TZ1.5d(iii):
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
-
19M.2.hl.TZ2.5f:
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
-
19M.1.hl.TZ2.26:
Where is the buffer region for the titration of a weak acid with a strong base?
- 19N.2.hl.TZ0.5a: A sample of ethanoic acid was titrated with sodium hydroxide solution, and the following pH curve...
- 19N.2.hl.TZ0.5b(i): Identify the most suitable indicator for the titration using section 22 of the data booklet.
-
19N.2.hl.TZ0.5b(ii):
Describe, using a suitable equation, how the buffer solution formed during the titration resists pH changes when a small amount of acid is added.
-
20N.2.hl.TZ0.5b(i):
Identify the major species, other than water and potassium ions, at these points.
- 20N.2.hl.TZ0.5b(ii): State a suitable indicator for this titration. Use section 22 of the data booklet
-
20N.2.hl.TZ0.5b(iii):
Suggest, giving a reason, which point on the curve is considered a buffer region.
- 21M.1.hl.TZ2.27: Which compound is acidic in aqueous solution? A. KBr B. CH3COONa C. NH4Cl D. Na2CO3
-
21M.2.hl.TZ1.8b:
Sketch the general shape of the variation of pH when 50 cm3 of 0.001 mol dm–3 NaOH (aq) is gradually added to 25 cm3 of 0.001 mol dm–3 CH3CH2COOH (aq).
- 21M.2.hl.TZ2.5d(i): Sketch the titration curve of methanoic acid with sodium hydroxide, showing how you would...
-
21M.2.hl.TZ2.5d(ii):
Identify an indicator that could be used for the titration in 5(d)(i), using section 22 of the data booklet.
-
21M.2.hl.TZ2.5f:
Identify if aqueous solutions of the following salts are acidic, basic, or neutral.
-
21N.2.hl.TZ0.11c:
Sketch the neutralisation curve obtained and label the equivalence point.
- 22M.1.hl.TZ1.27: In which set are the salts arranged in order of increasing pH? A. HCOONH4 < KBr < NH4Br...
- 22M.1.hl.TZ2.26: A weak base is titrated with a strong acid. Which value of pKb can be estimated from this...
- 22M.2.hl.TZ1.1d(ii): Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that...
- 22M.2.hl.TZ2.7a(iii): Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer...
-
22M.2.hl.TZ1.4c(iii):
An aqueous solution containing high concentrations of both NH3 and NH4+ acts as an acid-base buffer solution as a result of the equilibrium:
NH3 (aq) + H+ (aq) NH4+ (aq)
Referring to this equilibrium, outline why adding a small volume of strong acid would leave the pH of the buffer solution almost unchanged.
