| Date | May 2019 | Marks available | 1 | Reference code | 19M.1.hl.TZ2.16 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 16 | Adapted from | N/A |
Question
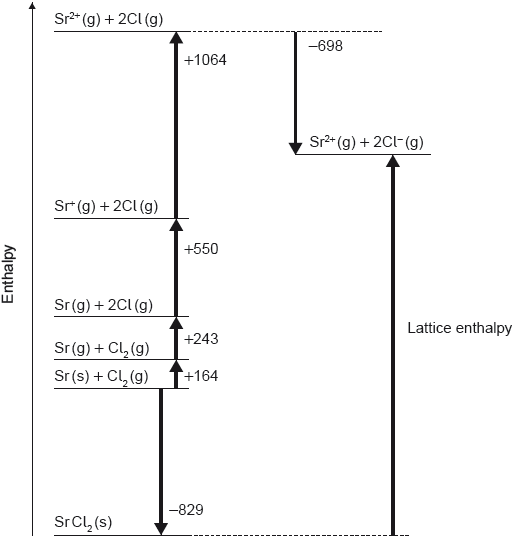
Which equation represents lattice enthalpy?
A. NaCl (g) → Na+ (g) + Cl− (g)
B. NaCl (s) → Na+ (g) + Cl− (g)
C. NaCl (s) → Na+ (aq) + Cl− (aq)
D. NaCl (s) → Na+ (s) + Cl− (s)
Markscheme
B
Examiners report
72 % of candidates chose the correct equation that represents lattice enthalpy. Many candidates chose A, where the ionic compound (NaCl) was gaseous, and others chose distractor C, where the ions produced were aqueous. The discrimination index for the question was quite high.