 Chemical reactions occur at different rates. An explosion may be a rapid chemical reaction, rusting is a much slower process. Kinetics is the study of the rates of reactions and the corresponding pathways that reactions may take.
Chemical reactions occur at different rates. An explosion may be a rapid chemical reaction, rusting is a much slower process. Kinetics is the study of the rates of reactions and the corresponding pathways that reactions may take.

Activation energy
Topic 16.2 A complex plot using the variation in rate with temperature can be used to find activation energy.

Collision theory and rates of reaction
Topic 6.1 Kinetic theory is the model that suggests that only colliding particles may react.
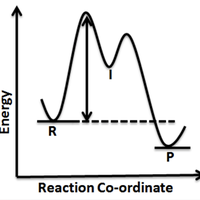
Rate expression and reaction mechanism
Topic 16.1 The rate expression (rate equation), determined experimentally, gives us information about possible reaction mechanisms.


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn