Topics 13.1 and 13.2
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
What is the overall charge, \(x\), on the cobalt (II) complex ion: [CoCl4]\(^x\)
The charge on the cobalt ion is 2+ (given by the Roman numerals, II). Each chloride ligand (Cl−) has a charge of 1−, so the overall charge will be 4− + 2+ = 2−.
(Charge is always expressed as number then sign, that is 2−, not −2.)
2− is therefore the correct answer.
Which of these statements is/are correct?
1: Ligands are Lewis bases
2: Zinc compounds are coloured
3: Titanium (II) compounds are paramagnetic
Ligands are molecules or negative ions that donate lone pair(s) of electrons to form coordinate (dative) bonds to a metal ion. Ligands are Lewis bases; metal ions are Lewis acids (electron pair donor/acceptor).
Zinc is not considered to be a transition element as its only stable ion is Zn2+ with a full d sub-level, so it does not form coloured compounds.
Paramagnetic substances have unpaired electrons (and behave as if they are weakly magnetic when an external magnetic field is applied). Titanium (II) ions have unpaired electrons in their electronic configuration (1s2 2s2 2p6 3s2 3p6 4s0 3d2). Remember that 4s electrons are gained first and lost first, and that all orbitals in a given sub-level are filled singly first, before electrons pair-up; the two d-electrons will be unpaired. Therefore, titanium (II) compounds are paramagnetic.
Thus 1 and 3 only is the correct answer.
What is the effect on d-orbital splitting and wavelength of light absorbed of a stronger ligand in an octahedral transition metal complex ion?
The spectrochemical series shows the increasing strength of ligands.
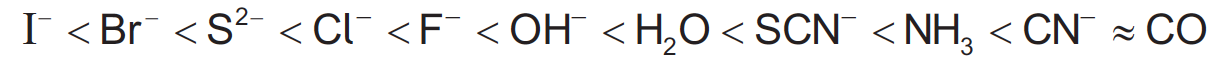
Stronger ligands cause a greater split in the d-orbitals, this means that the energy gap (ΔE) increases. However, energy of light is inversely proportional to wavelength, so as the energy gap increases the wavelength of light absorbed by the gap will decrease.
Therefore, d-orbital splitting increases and wavelength decreases is the correct answer.
Which of these statements about copper (I) (Cu+) and copper (II) (Cu2+) compounds is correct?
Substances with all electrons paired are diamagnetic – they are not magnetic, but may be slightly repelled by magnetic fields. Copper (I) ions (Cu+) have electronic configuration 1s2 2s2 2p6 3s2 3p6 4s0 3d10 (remember that 4s electrons are gained first and lost first), so copper (I) compounds are diamagnetic.
Paramagnetic substances have unpaired electrons (and behave as if they are weakly magnetic when an external magnetic field is applied). Copper (II) ions have unpaired electrons in their electronic configuration (1s2 2s2 2p6 3s2 3p6 4s0 3d9). Therefore, copper (II) compounds are paramagnetic.
Thus Copper (I) compounds are diamagnetic and copper (II) compounds are paramagnetic is the correct answer.
Which contributes to colour in transition metal complexes?
1: The splitting of the d-orbitals in energy.
2: The d-orbitals are partially filled with electrons.
3: Light is emitted when electrons fall between split d-orbitals.
Transition metal complexes are coloured because:
- Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies.
- The energy gap corresponds to wavelengths of visible light.
- Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed e.g. copper sulfate solution is blue – orange light is absorbed.
- It is necessary for d-orbitals to be partially-filled or electrons have no space to move.
Thus 1 and 2 only is the correct answer.
What is the oxidation state of the iron, and the overall charge on the complex ion, in the compound [Fe(H2O)4(OH)2]NO3?
The nitrate ion (NO3−) has a charge of 1−, so in order for the compound to be neutral, the overall charge on the complex ion must be 1+.
The charge on the iron ion is therefore 3+, since each hydroxide ligand (OH−) has a charge of 1− (the water ligands have no charge) and the overall charge on the complex ion is 1+ (3+ + 2− = 1+).
Charge is always expressed as number then sign, so the charge on the iron ion is 3+, but the oxidation state is +3.
Oxidation state +3; charge on the complex ion 1+ is therefore the correct answer.
Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies and the energy gap corresponds to wavelengths of visible light.
Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed.
Recalling the colour wheel:
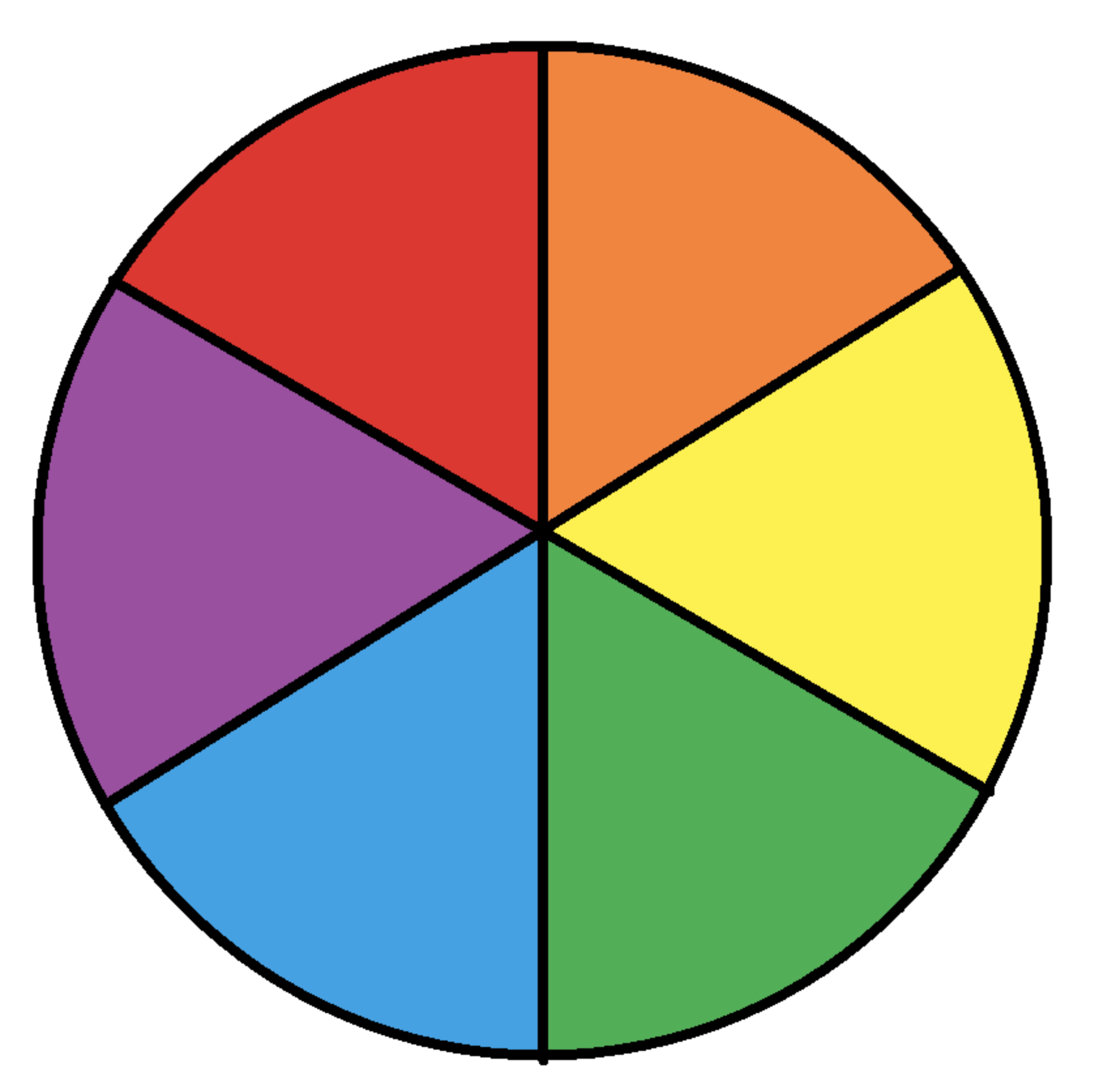
As a rule of thumb, the colour observed is the opposite of the (maximum frequency of light of the) colour absorbed. The observed colour orange has a complementary colour of absorbed light of blue, and blue light corresponds to the highest frequency/energy (lowest wavelength) of all the complementary colours (as given in the question).
Summary: Complementary colours are Orange→Blue, Violet→Yellow, Green→Red, Blue→Orange. And energy of light (absorbed): Blue > Yellow > Orange > Red (ROYGBIV)
Orange is thus the correct answer.
Part of the spectrochemical series is shown below:
Br− < F− < H2O < CN−
Which statement can be correctly deduced from the series?
Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies and the energy gap corresponds to wavelengths of visible light.
Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed.
The spectrochemical series indicates the strength of the ligands (I− the weakest; CN− and CO the strongest), with stronger ligands (better electron pair donors) causing greater d-orbital splitting.
![]()
The colour of complexes cannot be determined by the spectrochemical series, since colour also depends on the metal ion and its oxidation state.
Therefore the correct answer is CN− ligands cause greater d-orbital splitting than H2O.
Comparing [CuCl4]2− and [Cu(H2O)6]2+ which of these statements is/are correct?
1: [CuCl4]2− has greater d-orbital splitting because of the lower oxidation state of the copper
2: Both complexes are octahedral
3: The copper ion has electron configuration of [Ar]3d9 in both complexes
The [CuCl4]2− complex is tetrahedral, the chloride ligands have a 1− charge and so the copper ion is Cu2+.
The [Cu(H2O)6]2+ complex is octahedral, water ligands have no charge and so the copper ion is also Cu2+.
The copper ion therefore has electron configuration of [Ar]3d9 in both complexes.
The degree of d-orbital splitting, and hence the colour of the complex, varies with the metal and oxidation state of the transition metal ion and with the strength of the ligand. However, the oxidation state of the copper is the same in both complexes (+2) so statement 1 is incorrect.
Thus 3 only is the correct answer.
What is the coordination number and the oxidation state of the iron ion in [Fe(CN)6]3– ?
The CN– ligand is a cyanide ion and has a 1− charge.
Each CN– forms one coordinate bond to the iron ion, and there are six ligands. Therefore there are a total of six coordinate bonds into the iron centre - the coordination number is 6.
The overall charge on the complex is 3−, so the iron ion must have a charge of 3+ (6− + 3+ = 3−). Therefore the oxidation state of the iron is +3.
Therefore the correct answer is Coordination number 6 ; oxidation state +3.
How much of Periodicity AHL (HL only) paper 1 questions have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn