Atomos is an ancient Greek word meaning 'indivisible' or 'cannot be cut'. Atoms are the building blocks of matter; they are the smallest particles that when combined together have the properties of a particular chemical element or compound
This section of the International Baccalaureate Diploma Programme Chemistry revision and study guide focuses on sections 2 (Core) and 12 (Additional Higher Level) of the IBDP Chemistry course: Find links to the chemistry immediately below.
The nuclear atom
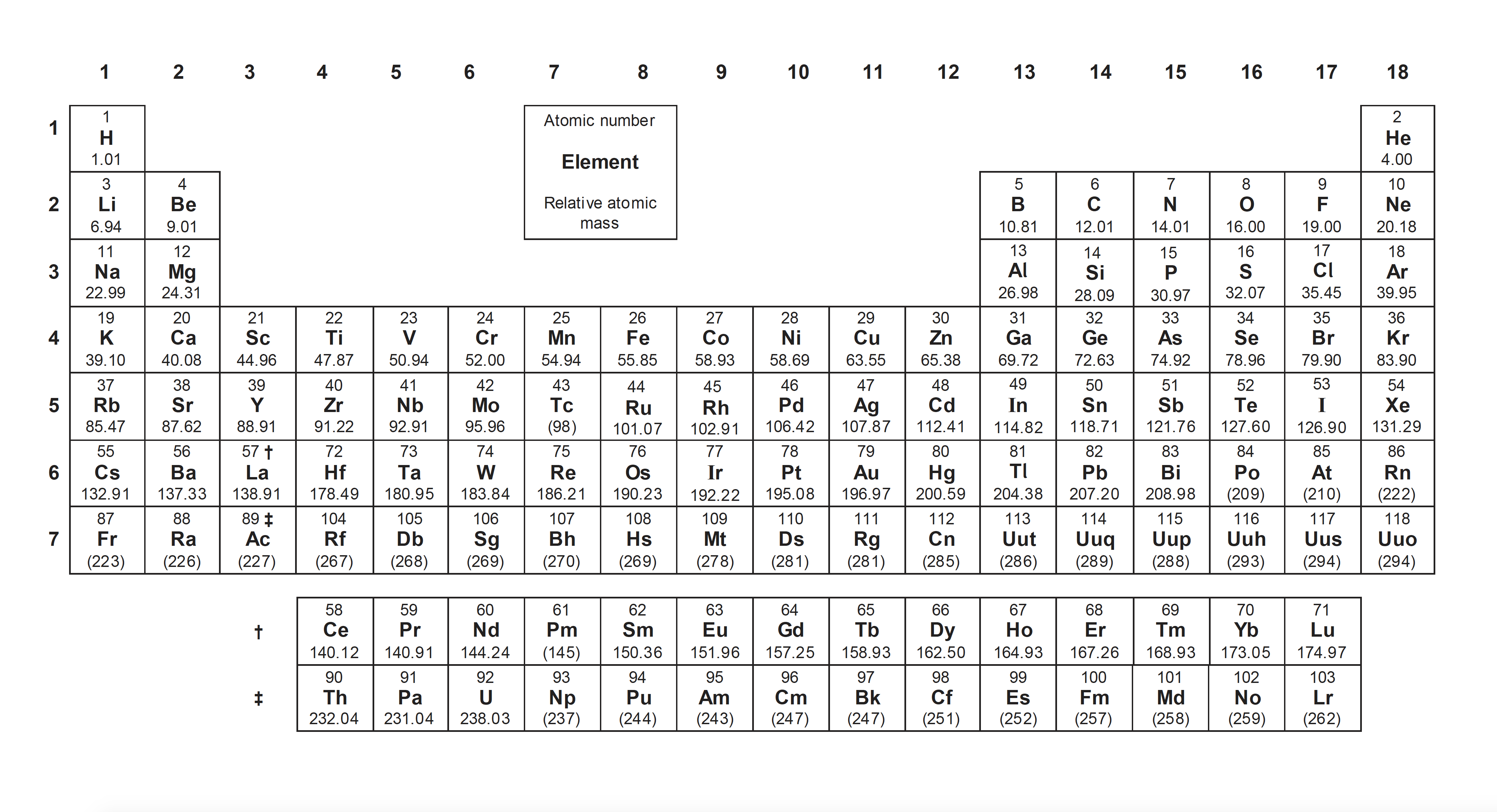
This section covers the foundations of atomic structure and explains the numerical values given on the periodic table. It is a relatively straightforward part of the course in terms of concepts, but a firm grip of the specific terms and calculations are needed before moving on.
Electron configuration
Electron configuration, that is the distribution or arrangement of electrons in the atom, can be described by the Bohr model, or the quantum mechanical model, of the atom. The Bohr model is simpler and the quantum mechanical model more complex, but both are similar in some ways.
Electrons in atoms
First ionisation energies provide evidence for our quantum mechanical model of the atom. This is a challenging section of the course; abstract concepts and lots of specific terms, combined later with mathematics create a real challenge. But you will get your head around it eventually!
What's in an Atom?
Protons, neutrons and electrons are sub-atomic particles that together make up atoms. Protons and neutrons are found in the centre (nucleus) of an atom and electrons are found in the space around the nucleus. Read more: The nuclear atom
What are mass and charge of a proton, neutron and electron?
Protons are positively charged, and have a mass of 1 unit; neutrons are neutral and have a mass of 1 unit; electrons are negatively charged and have negligible mass. See the revision cards: The nuclear atom
What's the definition of an isotope?
Isotopes are atoms of the same element (same number of protons) with a different mass number (different number of neutrons). See the revision cards: The nuclear atom
What does a mass spectrum show?
A mass spectrum is a chart showing the mass numbers of chemical species (atoms, molecules, fragments of molecules; all of which have been turned into ions so that they can be detected). Read more: The nuclear atom and Spectroscopic identification I
What does Bohr's model explain?
The Bohr model shows electrons in shells (or energy levels) around the nucleus with the number of electrons in the outermost shell matching the main group number (old Roman numerals) on the periodic table. Watch the video: Electron configuration
What is the quantum mechanical model of the atom?
The quantum mechanical model of the atom is a development of the Bohr model, showing the electrons in energy levels, but also showing finer detail with the electrons in sub-levels and orbitals. The quantum mechanical model of the atom is reflected in the arrangement of elements in the periodic table! Watch the videos: Electron configuration
What is an orbital in chemistry?
A three-dimensional space in which a maximum of two electrons may be found. Read more: Electron configuration
What is the emission spectrum of hydrogen?
The emission spectrum of hydrogen is a line spectrum − showing specific frequencies of light − indicating the energy associated with particular transitions (movement) of electrons between energy levels. The spectrum provides evidence for the quantum mechanical model of the atom. Watch the video: Electron configuration
What is first ionisation energy?
First ionisation energy for the an atom of an element is an indication of the energy needed to remove the outermost electron in the atom (more specifically one electron from every atom in one mole of atoms in the gas phase). So first ionisation energies tell us the relative energies of electrons; that is the energy of the orbital, sub-level and energy level in which the electron sits. Watch the video: Electron configuration
Electron configuration
Topic 2.2 Introducing a new model of the atom, which is a development of Bohr's model.
Electrons in atoms
Topic 12.1 Exploring what ionisation energies can tell us about the nature of the atom.

The nuclear atom
Topic 2.1 Introducing the principle of sub-atomic particles.


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn