Topics 3.1 and 3.2
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which oxide when added to water will give the solution with the lowest pH?
This question involves knowledge of structure and bonding as well as the acidic and basic nature of oxides.
Metal oxides (MgO and Na2O), if they react with water at all, will give alkaline solutions that therefore have a pH higher than 7.
Non-metal oxides may react with water to give acidic solutions that therefore have a pH lower than 7. However, SiO2 (silica) is a giant covalent structure analogous to diamond, and does not react with water (sand is impure SiO2). Sulfur trioxide, SO3, will react (very exothermically) in water to give an acidic solution of sulfuric acid (H2SO4).
SO3 is therefore the correct answer.
What is the correct trend in melting point going down group 1 and group 17?
Group 1 metals have metallic bonding and therefore giant structures. Melting points are relatively low for metals as the atoms are large and form 1+ ions so the attraction between the nucleus and ‘sea of electrons’ is not as strong as in other metals. Nonetheless strong metallic bonds must be broken. The melting points (values in data book) become lower down the group because of the increasing atomic/ion size leads to less attraction..
Group 17 non-metals; the halogens, have covalent bonding and simple molecular structures. The atoms get bigger down the group, so the diatomic halogen molecules get bigger down the group. These non-polar molecules have weak London dispersion forces that must be broken. The melting points (data book) become higher down the group as London dispersion forces get stronger as the number of electrons/molar mass of a molecule increases.
Thus, Decrease down group 1 and increase down group 17 is the correct answer.
In which block are the elements (atomic numbers in brackets) Nd (60), Zn (30) and Xe (54) respectively?
Which of the following statements is/are true with regard to metals?
1: Metals tend to form acidic oxides.
2: Metals tend to be good conductors of electricity
3: Metals tend to have lower ionisation energies than non-metals
Metals tend to form basic (alkaline) oxides, not acidic oxides, so statement 1 is false.
Metals' outer electrons are delocalised and they have giant structures. Metals are often: good conductors of electricity and heat; high in melting and boiling point; malleable; ductile; sonorous; lustrous (shiny). Therefore statement 2 is true.
Metals have low electronegativity and tend to lose electrons when they react. The trend in ionisation energy across a period (from metals to non-metals) is a general increase. Therefore statement 3 is true.
Thus 2 and 3 only is the correct answer.
Which of the equations below represents the first electron affinity of chlorine?
First electron affinity and second electron affinity are definitions that need to be learned:
First electron affinity is the enthalpy change when one electron is gained by each atom in one mole of gaseous atoms.
Second electron affinity is the enthalpy change when one electron is gained by each 1− ion in one mole of gaseous ions.
Thus Cl(g) + e− → Cl−(g) is the correct answer, as this is the first electron affinity of chlorine.
Which series represents atoms in order of decreasing atomic radius?
Across any period there is a general decrease in atomic radii due to increasing nuclear charge and electrons filling the same energy level. The electrons are in the same energy level, but the nuclear charge increases (number of protons increases), increasing the attraction between the nucleus and the electrons and pulling them in more tightly/closer to the nucleus. Overall the attraction between the outer electrons and the nucleus outweighs the increasing electron-electron repulsion.
Down any group there is a general increase in atomic radii due to electrons filling energy levels that are further from the nucleus making the atoms bigger, despite the increase in nuclear charge.
Thus K > Na > Al > Si is the correct answer. K is lower than sodium in group 1, so Na will have a smaller atom radius, and Al and Si are elements further across period 3 than Na, so will have decreasing atomic radii.
Which oxides, when added to water, will give an alkaline solution?
1: P4O10
2: SO3
3: CaO
Metal oxides (CaO), if they react with water at all, will give alkaline solutions (this does react).
Non-metal oxides (P4O10 and SO3), if they react with water at all, will give acidic solutions (both of these do react).
3 only is therefore the correct answer.
Which statement about trends is true in both groups 2 and 18?
Group 2 are metals, so have metallic bonding and therefore giant structures. The melting and boiling points become lower down the group because of the increasing atomic/ion size leads to less attraction.
Group 18 are non-metals; the noble gases, and exist as atomic gases. The melting and boiling points become higher down the group as London dispersion forces get stronger as the number of electrons/molar mass of the atoms increases.
Electronegativities decrease down all groups (and increase across periods).
Ionisation energies decrease down all groups (and show a general increase across periods).
Thus Ionisation energies decrease is the correct answer.
Which trends are generally correct across period 2 (Li to Ne)?
1: Atomic radius decreases
2: Boiling point increases
3: 1st ionisation energy increases
Metals and Non-metals (or metalloids) with giant structures (Li, Be, B and C) have high melting/boiling points; strong covalent or metallic bonds are broken. Non-metals with simple molecular structures (N2, O2, F2) or gaseous atoms (Ne) have low melting/boiling points; only intermolecular forces need to be broken. So statement 2 is false as boiling point will peak at C (in the middle) across period 2.
Across any period there is a general decrease in atomic radii due to increasing nuclear charge and electrons filling the same energy level. The electrons are in the same energy level, but the nuclear charge increases (number of protons increases), increasing the attraction between the nucleus and the electrons and pulling them in more tightly/closer to the nucleus. Overall the attraction between the outer electrons and the nucleus outweighs the increasing electron-electron repulsion. Statement 1 is correct.
Across any period there is a general increase in 1st ionization energy due to increasing nuclear charge and electrons filling the same energy level. So the outermost electron becomes subject to greater attraction and more difficult to remove. Statement 3 is correct.
Thus 1 and 3 only is the correct answer.
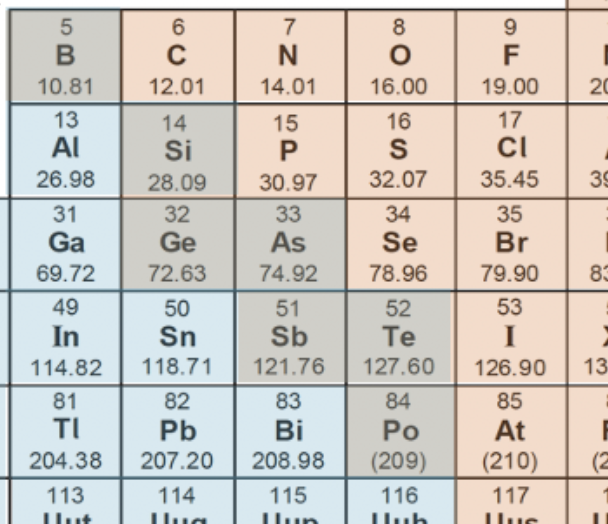
Which element is not a metalloid?
Metalloids lay on the diagonal between metals and non-metals. Beginning with Boron each successive element on the diagonal, and the one underneath it, are considered to be metalloids, ending in Astatine (which is not considered to be a metalloid):
Which correctly describes the reaction between sodium and excess water?
Group 1 metals react exothermically with water to give strongly alkaline solutions of the metal hydroxide and hydrogen gas, e.g. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Therefore 'The reaction is exothermic and the products are sodium hydroxide and hydrogen' is the correct answer.
What is the trend in electronegativity across period 2 and down group 17?
The most electronegative element is Fluorine (4.0) and the least is Francium (0.7). Therefore, electronegativity increases across all periods and up all groups. You don't need to know the values, but you do need to know the trend.
Therefore the correct answer is 'Electronegativity increases across period 2 and decreases down group 17'.
How much of Periodicity core (SL and HL) paper 1 questions have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn